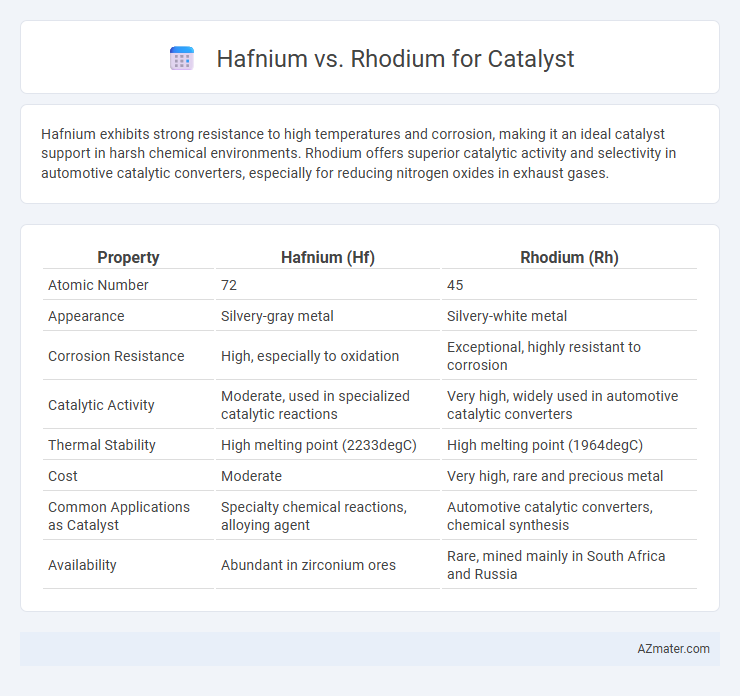
Hafnium exhibits strong resistance to high temperatures and corrosion, making it an ideal catalyst support in harsh chemical environments. Rhodium offers superior catalytic activity and selectivity in automotive catalytic converters, especially for reducing nitrogen oxides in exhaust gases.
Table of Comparison
| Property | Hafnium (Hf) | Rhodium (Rh) |
|---|---|---|
| Atomic Number | 72 | 45 |
| Appearance | Silvery-gray metal | Silvery-white metal |
| Corrosion Resistance | High, especially to oxidation | Exceptional, highly resistant to corrosion |
| Catalytic Activity | Moderate, used in specialized catalytic reactions | Very high, widely used in automotive catalytic converters |
| Thermal Stability | High melting point (2233degC) | High melting point (1964degC) |
| Cost | Moderate | Very high, rare and precious metal |
| Common Applications as Catalyst | Specialty chemical reactions, alloying agent | Automotive catalytic converters, chemical synthesis |
| Availability | Abundant in zirconium ores | Rare, mined mainly in South Africa and Russia |
Introduction to Hafnium and Rhodium as Catalysts
Hafnium and rhodium are transition metals frequently employed as catalysts in various chemical reactions, with rhodium widely recognized for its exceptional catalytic efficiency in hydrogenation and automotive catalytic converters. Hafnium, though less common, offers unique catalytic properties due to its high melting point and resistance to corrosion, making it valuable in high-temperature applications. The catalytic performance of rhodium typically surpasses hafnium in terms of activity and selectivity, but hafnium's stability under extreme conditions presents distinct advantages in specialized catalytic processes.
Chemical Properties Relevant to Catalysis
Hafnium exhibits strong resistance to corrosion and high thermal stability, making it suitable for catalytic processes requiring durability under extreme conditions, although it has relatively low catalytic activity compared to noble metals. Rhodium, characterized by its exceptional catalytic efficiency, particularly in oxidation and hydrogenation reactions, offers superior chemical inertness and resistance to poisoning, which enhances catalyst longevity and selectivity. The distinct electronic configurations and surface chemistries of Rhodium contribute to its widespread use in automotive catalytic converters and fine chemical synthesis, whereas Hafnium's applications are more niche, often limited by its lower catalytic surface area and reactivity.
Catalyst Efficiency: Hafnium vs Rhodium
Hafnium exhibits moderate catalytic efficiency, often used in high-temperature and corrosion-resistant catalyst applications due to its stability and ability to form stable carbides. Rhodium outperforms hafnium in catalytic efficiency, especially in automotive catalytic converters and industrial processes, because of its exceptional ability to facilitate oxidation and reduction reactions at lower temperatures. The superior catalytic properties of rhodium result in enhanced reaction rates and selectivity, making it the preferred choice for catalysts requiring high performance and durability.
Cost Comparison and Economic Feasibility
Hafnium catalysts generally exhibit lower market prices than rhodium, making them a cost-efficient option for large-scale industrial applications. Rhodium's scarcity and high demand in automotive catalytic converters significantly drive its price, impacting economic feasibility for extensive usage. Evaluating catalyst performance relative to cost, hafnium presents a promising balance of affordability and catalytic efficiency, enhancing economic viability in various chemical processes.
Stability and Durability in Catalytic Reactions
Hafnium exhibits superior thermal stability and corrosion resistance in catalytic reactions, making it a robust choice for high-temperature processes. Rhodium offers exceptional catalytic activity and resistance to poisoning, but its stability under prolonged high-temperature conditions can be less reliable compared to hafnium. The durability of hafnium catalysts in harsh environments often results in longer operational lifespans, whereas rhodium catalysts may require more frequent regeneration or replacement.
Environmental Impact and Sustainability
Hafnium and Rhodium catalysts differ significantly in environmental impact and sustainability. Rhodium, a rare and highly valuable platinum-group metal, offers exceptional catalytic efficiency but poses sustainability challenges due to its limited availability and intensive mining processes. Hafnium, while less common in catalytic applications, presents a potentially more sustainable alternative with lower toxicity and increased abundance, supporting greener industrial practices.
Industrial Applications and Use Cases
Hafnium and rhodium catalysts exhibit distinct industrial applications due to their unique chemical properties and cost profiles. Rhodium, valued for its exceptional resistance to corrosion and high catalytic activity, is predominantly used in automotive catalytic converters and chemical synthesis, including the production of acetic acid and nitric acid. Hafnium catalysts, though less common, find specialized use in high-temperature processes and nuclear reactors, leveraging their superior thermal stability and neutron absorption characteristics for industrial applications such as desulfurization and alloy production.
Availability and Supply Chain Considerations
Hafnium's availability is more limited due to its occurrence primarily as a byproduct of zirconium refining, leading to supply constraints and higher extraction costs. Rhodium, though rare, benefits from a more established mining and recycling infrastructure within the platinum group metals market, ensuring relatively steadier supply chains. Supply chain disruptions for both metals are influenced by geopolitical factors and market demand, but rhodium's integration in automotive catalytic converters drives significant investment in securing its availability.
Future Trends in Catalyst Innovation
Hafnium-based catalysts are gaining attention for their high thermal stability and resistance to corrosion, making them ideal for harsh chemical processes. Rhodium catalysts continue to dominate in automotive emission control due to their exceptional efficiency in reducing nitrogen oxides and hydrocarbons. Future trends indicate a shift towards hafnium alloys and nano-structured catalysts that combine the durability of hafnium with rhodium's catalytic activity for enhanced performance and sustainability in green technology applications.
Conclusion: Selecting the Ideal Catalyst
Hafnium catalysts offer exceptional thermal stability and resistance to corrosion, making them suitable for high-temperature industrial processes. Rhodium catalysts provide superior activity and selectivity in hydrogenation and carbonylation reactions, delivering efficient conversion rates and reduced by-products. Selecting the ideal catalyst depends on specific application requirements, balancing Hafnium's durability with Rhodium's catalytic efficiency to optimize performance and cost-effectiveness.
Infographic: Hafnium vs Rhodium for Catalyst
 azmater.com
azmater.com