Hafnium offers exceptional corrosion resistance and high melting point, making it ideal for advanced nuclear reactors and aerospace applications. Francium, being highly radioactive and scarce, is primarily used in specialized atomic research rather than practical material applications.
Table of Comparison
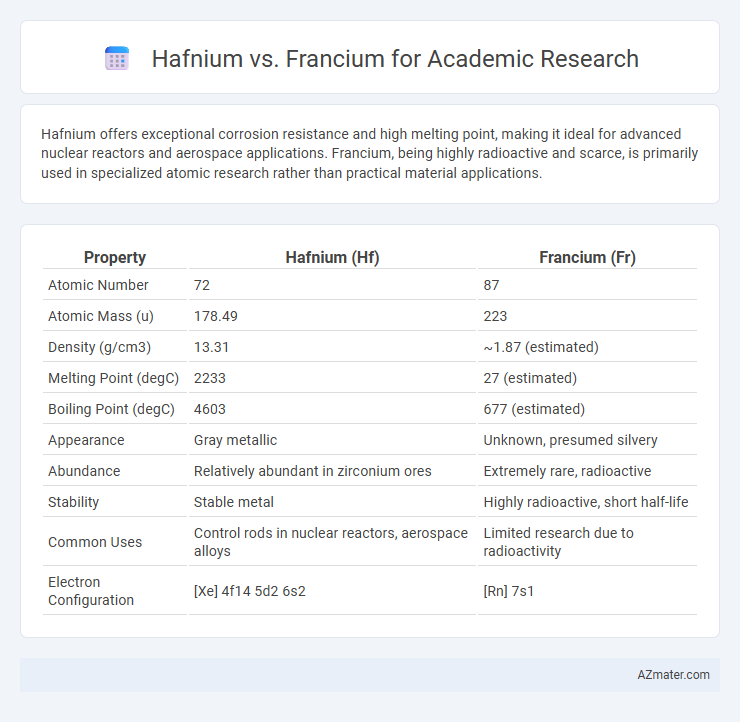
| Property | Hafnium (Hf) | Francium (Fr) |
|---|---|---|
| Atomic Number | 72 | 87 |
| Atomic Mass (u) | 178.49 | 223 |
| Density (g/cm3) | 13.31 | ~1.87 (estimated) |
| Melting Point (degC) | 2233 | 27 (estimated) |
| Boiling Point (degC) | 4603 | 677 (estimated) |
| Appearance | Gray metallic | Unknown, presumed silvery |
| Abundance | Relatively abundant in zirconium ores | Extremely rare, radioactive |
| Stability | Stable metal | Highly radioactive, short half-life |
| Common Uses | Control rods in nuclear reactors, aerospace alloys | Limited research due to radioactivity |
| Electron Configuration | [Xe] 4f14 5d2 6s2 | [Rn] 7s1 |
Introduction to Hafnium and Francium
Hafnium (Hf), atomic number 72, is a transition metal known for its high melting point and corrosion resistance, making it valuable in nuclear reactors and aerospace applications. Francium (Fr), atomic number 87, is a highly radioactive alkali metal with limited availability and extreme instability, which restricts its use primarily to scientific research in nuclear physics and chemistry. The stark contrast in their physical and chemical properties drives distinct research focuses, with hafnium's utility in material science and francium's role in fundamental atomic studies.
Elemental Properties: Hafnium vs Francium
Hafnium (Hf), atomic number 72, is a transition metal with high melting point (2233degC) and excellent corrosion resistance, making it valuable in nuclear reactors and aerospace applications. Francium (Fr), atomic number 87, is a highly radioactive alkali metal with a short half-life, existing only in trace amounts and exhibiting extreme instability. The stark contrast in their elemental properties--Hafnium's durability and Francium's radioactivity--defines their divergent roles and challenges in academic research.
Occurrence and Natural Abundance
Hafnium occurs naturally primarily in zirconium ores with an abundance of approximately 5.8 ppm in the Earth's crust, making it relatively accessible for academic research. Francium is extremely rare, with its natural occurrence limited to trace amounts produced by the decay of actinium and trace uranium and thorium, yielding an average abundance of less than 1 part per trillion. The stark contrast in availability significantly influences the feasibility and scope of research involving these elements, with hafnium being widely studied due to its stable isotopes and accessibility, while francium's radioactivity and scarcity restrict practical research applications.
Extraction and Preparation Methods
Hafnium is primarily extracted from zirconium ores through solvent extraction and ion-exchange methods, leveraging its chemical similarity to zirconium for separation. Francium, being highly radioactive and extremely rare, is typically produced synthetically in particle accelerators via the bombardment of thorium or uranium targets, with its isolation relying on rapid chemical separation techniques. The complexity and scarcity of francium limit its preparation to small-scale, short-lived samples, whereas hafnium's more abundant and stable nature allows for extensive extraction and purification processes suitable for academic research.
Chemical Reactivity and Stability
Hafnium, a transition metal with atomic number 72, exhibits high chemical stability and resistance to corrosion, making it ideal for academic research in materials science and nuclear applications. Francium, an alkali metal with atomic number 87, is extremely rare and highly reactive, displaying rapid oxidation and short half-life, which significantly limits its practical study and use in chemical research. The stark contrast in stability and reactivity between hafnium and francium defines their unique roles, with hafnium offering predictable chemical behavior and francium presenting challenges due to its radioactivity and fleeting existence.
Applications in Academic Research
Hafnium's unique properties, such as its high melting point and neutron absorption capabilities, make it invaluable in nuclear reactor research and advanced materials science studies. Francium's extreme radioactivity and scarcity limit its practical applications, but it is crucial for atomic structure experiments and fundamental physics research on heavy alkali metals. Academic research benefits from Hafnium's stability in creating durable alloys, while Francium offers insights into relativistic effects and weak nuclear interactions.
Safety and Handling Considerations
Hafnium, a transition metal with atomic number 72, is relatively safe to handle in controlled laboratory environments due to its stable chemical properties and low radioactivity. Francium, atomic number 87, is highly radioactive and exists only in trace amounts, making it extremely hazardous and impractical for routine academic research or handling without specialized containment facilities. The significant radiological risks and scarcity of francium necessitate stringent safety protocols, whereas hafnium offers more manageable handling conditions with established industrial and research applications.
Analytical Techniques for Study
Hafnium and francium present distinct challenges in analytical techniques due to their differing chemical properties and stability; hafnium's robust nature allows for precise characterization using X-ray fluorescence (XRF) and inductively coupled plasma mass spectrometry (ICP-MS). Francium's extreme radioactivity and scarcity necessitate specialized detection methods like alpha spectroscopy and rapid decay measurement in controlled environments. Research into hafnium benefits from advanced electron microscopy and neutron activation analysis, while francium studies rely heavily on atomic trapping and laser spectroscopy to observe its short-lived isotopes.
Recent Developments and Case Studies
Recent research on hafnium explores its robust thermal properties and neutron absorption capabilities, making it valuable in nuclear reactor control and aerospace applications, with case studies highlighting its use in advanced semiconductor devices and corrosion-resistant coatings. Francium, with its extreme radioactivity and scarcity, remains challenging to study, but recent developments utilize sophisticated trapping techniques in atomic physics experiments to investigate fundamental symmetries and nuclear decay processes. Comparative analyses emphasize hafnium's practical applications in material science versus francium's role in theoretical physics, highlighting divergent pathways for academic research focus.
Future Prospects in Scientific Research
Hafnium, with its stable isotopes and superior neutron absorption properties, holds significant potential for advancing nuclear research and materials science applications, particularly in next-generation reactor designs and semiconductor technology. Francium, despite its rarity and high radioactivity, offers intriguing prospects in atomic physics due to its heavy alkali metal properties, enabling precision measurements that could enhance understanding of fundamental symmetries and weak interactions. The future of scientific research will likely see hafnium's practical applications expanding in energy and electronics, while francium remains primarily a subject of fundamental theoretical investigations due to challenges in production and handling.
Infographic: Hafnium vs Francium for Academic Research
 azmater.com
azmater.com