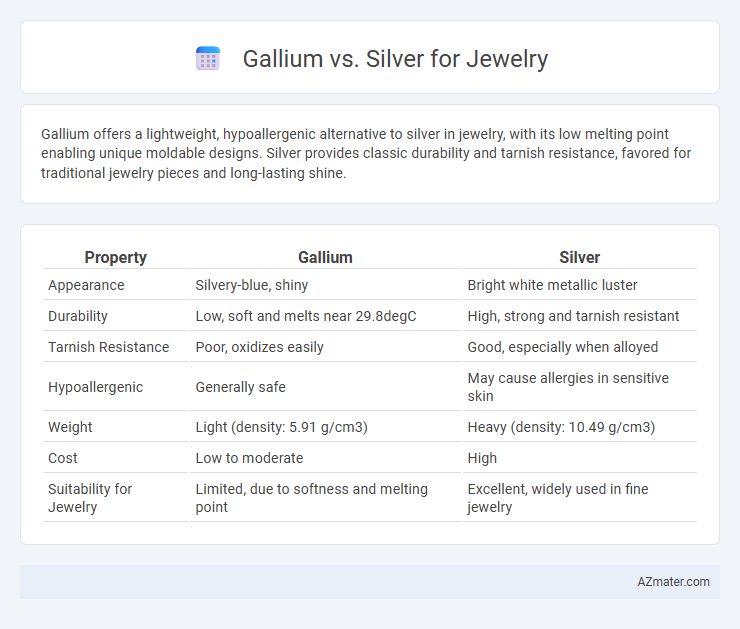
Gallium offers a lightweight, hypoallergenic alternative to silver in jewelry, with its low melting point enabling unique moldable designs. Silver provides classic durability and tarnish resistance, favored for traditional jewelry pieces and long-lasting shine.
Table of Comparison
| Property | Gallium | Silver |
|---|---|---|
| Appearance | Silvery-blue, shiny | Bright white metallic luster |
| Durability | Low, soft and melts near 29.8degC | High, strong and tarnish resistant |
| Tarnish Resistance | Poor, oxidizes easily | Good, especially when alloyed |
| Hypoallergenic | Generally safe | May cause allergies in sensitive skin |
| Weight | Light (density: 5.91 g/cm3) | Heavy (density: 10.49 g/cm3) |
| Cost | Low to moderate | High |
| Suitability for Jewelry | Limited, due to softness and melting point | Excellent, widely used in fine jewelry |
Introduction to Gallium and Silver in Jewelry
Gallium, a soft, silvery metal with a melting point near 29.8degC, offers unique properties for contemporary jewelry, including malleability and hypoallergenic qualities. Silver, a traditional and highly valued metal in jewelry, is prized for its luster, durability, and affordability, making it a popular choice for rings, necklaces, and bracelets. Both metals serve distinct purposes in jewelry design, with gallium often used in experimental or artistic pieces and silver maintaining widespread appeal and classic elegance.
Metal Properties: Gallium vs Silver
Gallium boasts a melting point of approximately 29.76degC, making it significantly softer and less durable compared to silver, which has a melting point of 961.8degC and is known for its strength and resistance to tarnish. Silver's higher density and hardness provide longevity for jewelry, while gallium's low melting point renders it impractical for everyday wear. The conductivity and luster of silver combined with its corrosion resistance make it a superior choice for jewelry metals compared to the more reactive and fragile nature of gallium.
Durability and Wearability Comparison
Gallium, known for its low melting point and softness, lacks the durability required for everyday jewelry compared to silver, which boasts higher hardness and resistance to scratching. Silver, specifically sterling silver (92.5% purity), offers excellent wearability with its sturdiness and tarnish resistance when properly cared for. Gallium's tendency to oxidize and deform under heat limits its practical use in jewelry, while silver remains a reliable choice for long-lasting, wearable pieces.
Aesthetic Appeal: Color, Shine, and Texture
Gallium jewelry offers a unique silvery-white hue with a liquid metal sheen that contrasts the classic bright white and highly reflective surface of silver. Its smooth, almost glass-like texture enhances modern and futuristic designs, while silver provides a more traditional, warm luster favored in vintage or classic styles. The vibrant shine of silver tends to maintain a polished finish longer, whereas gallium's softer metallic glow is ideal for avant-garde aesthetics seeking innovation in color and surface smoothness.
Hypoallergenic and Skin Safety Factors
Gallium offers superior hypoallergenic properties compared to silver, making it an excellent choice for sensitive skin and reducing the risk of allergic reactions and irritation. Silver, especially sterling variants containing nickel or other metals, can cause allergies and skin discoloration in some individuals. Gallium's biocompatibility and low reactivity enhance skin safety, ensuring long-lasting, comfortable wear for jewelry aficionados with delicate skin.
Cost and Market Value Differences
Gallium is significantly cheaper than silver due to its abundance and lower demand in the jewelry market, with prices approximately $0.5 per gram compared to silver's average of $0.8 per gram. Silver holds higher market value driven by its widespread use, historical significance, and established desirability in jewelry, making it a more prestigious and valuable material. The cost disparity influences consumer preference, with silver being favored for investment and luxury pieces, while gallium remains niche and experimental.
Workability for Jewelry Designers
Gallium offers exceptional workability for jewelry designers due to its low melting point of 29.76degC, allowing for easy shaping and molding without specialized equipment. Silver, with a higher melting point of 961.8degC, requires intense heat and traditional metalworking techniques such as soldering and casting, demanding more skill and tools. The softness and malleability of gallium enable intricate designs and prototypes, while silver provides durability and timeless appeal in finished pieces.
Environmental Impact and Sustainability
Gallium offers a more sustainable alternative to silver in jewelry production due to its lower environmental footprint and abundance in bauxite and zinc ores, reducing mining impacts associated with silver extraction. Silver mining generates significant ecological disturbance, including habitat destruction and water contamination from cyanide and mercury use, whereas gallium recovery, often as a byproduct of existing mining operations, minimizes additional environmental harm. Choosing gallium over silver supports circular economy principles by utilizing industrial byproducts and decreasing reliance on intensive mining practices, enhancing the sustainability profile of jewelry manufacturing.
Maintenance and Care Considerations
Gallium jewelry requires careful handling due to its softness and low melting point, making it prone to scratches and deformation, unlike silver which is much more durable and scratch-resistant. Silver jewelry demands regular polishing to prevent tarnish caused by oxidation, whereas gallium is less susceptible to tarnishing but may need protection from extreme temperatures and harsh chemicals. Both metals require gentle cleaning methods, but silver can withstand stronger cleaning agents, while gallium benefits from mild, non-abrasive care to maintain its appearance.
Trends and Future Prospects in Jewelry
Gallium's emerging role in jewelry design stems from its unique liquid metal properties and ability to form innovative, customizable pieces, contrasting with silver's traditional appeal and high conductivity. Trends show a growing interest in gallium-based jewelry for futuristic, adaptive aesthetics, while silver remains favored for its timeless elegance and affordability. Future prospects highlight gallium's potential in smart jewelry technology and sustainable production, suggesting a complementary presence alongside silver in evolving market demands.
Infographic: Gallium vs Silver for Jewelry
 azmater.com
azmater.com