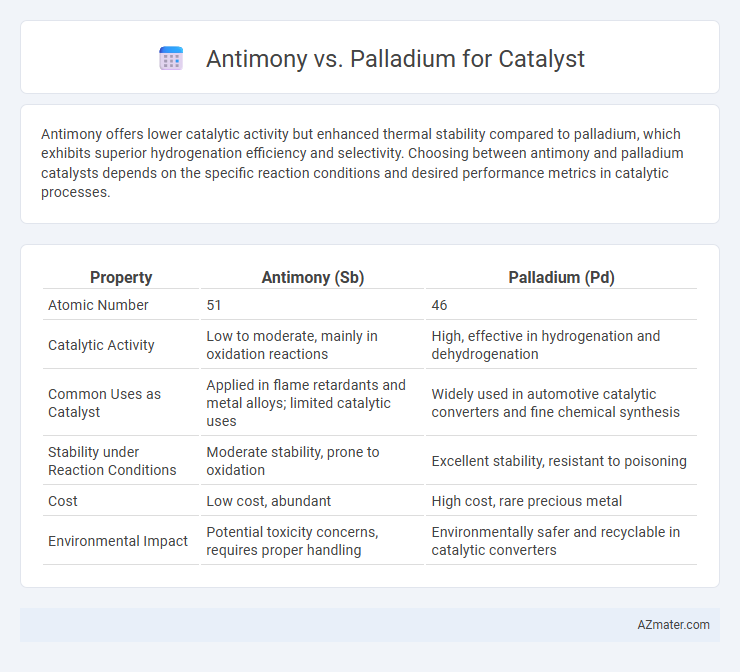
Antimony offers lower catalytic activity but enhanced thermal stability compared to palladium, which exhibits superior hydrogenation efficiency and selectivity. Choosing between antimony and palladium catalysts depends on the specific reaction conditions and desired performance metrics in catalytic processes.
Table of Comparison
| Property | Antimony (Sb) | Palladium (Pd) |
|---|---|---|
| Atomic Number | 51 | 46 |
| Catalytic Activity | Low to moderate, mainly in oxidation reactions | High, effective in hydrogenation and dehydrogenation |
| Common Uses as Catalyst | Applied in flame retardants and metal alloys; limited catalytic uses | Widely used in automotive catalytic converters and fine chemical synthesis |
| Stability under Reaction Conditions | Moderate stability, prone to oxidation | Excellent stability, resistant to poisoning |
| Cost | Low cost, abundant | High cost, rare precious metal |
| Environmental Impact | Potential toxicity concerns, requires proper handling | Environmentally safer and recyclable in catalytic converters |
Introduction to Antimony and Palladium Catalysts
Antimony catalysts are widely used for their ability to enhance reactions in organic synthesis, particularly in the production of polyethylene terephthalate (PET) and flame retardants, due to their thermal stability and oxidation resistance. Palladium catalysts are highly valued for their exceptional catalytic activity in hydrogenation, carbon-carbon coupling reactions, and automotive catalytic converters, owing to their ability to facilitate electron transfer and bond activation. Both metals offer unique catalytic properties, with antimony primarily favored in polymerization and flame retardancy, while palladium excels in fine chemical synthesis and environmental applications.
Chemical Properties Relevant to Catalysis
Antimony exhibits semimetallic properties with moderate electronegativity and forms stable compounds that can influence catalytic selectivity by modifying electronic environments. Palladium, a transition metal with high catalytic activity, excels in facilitating hydrogenation and carbon-carbon coupling reactions due to its variable oxidation states and ability to adsorb hydrogen effectively. The distinct electronic structures of antimony and palladium dictate their roles in catalysis, where palladium's d-orbitals enable versatile bond activation, whereas antimony often serves as a modifier in bimetallic catalysts to enhance performance.
Historical Applications in Catalysis
Antimony has been historically utilized in catalytic processes such as the production of polyethylene terephthalate (PET), where it acts as a polycondensation catalyst to enhance polymerization efficiency. Palladium's catalytic role dates back to the early 20th century, prominently in hydrogenation and carbon-carbon coupling reactions like the Suzuki and Heck reactions, revolutionizing organic synthesis. The catalytic effectiveness of palladium in forming carbon-carbon bonds made it indispensable in pharmaceutical and fine chemical industries, whereas antimony's applications remained largely confined to polymer production and flame retardancy.
Mechanisms of Catalytic Action
Antimony functions as a catalyst mainly by altering the electronic properties of the active site, thereby enhancing adsorption and activation of reactant molecules through its variable oxidation states. Palladium exhibits catalytic activity via the formation of metal-hydride and metal-carbon intermediates, facilitating hydrogenation and carbon-carbon bond formation through oxidative addition and reductive elimination cycles. The differing mechanisms arise from antimony's semimetal characteristics influencing electron density distribution, while palladium's d-orbital electron configuration enables reversible metal-substrate interactions crucial for catalytic turnover.
Efficiency and Selectivity in Reactions
Antimony catalysts exhibit unique electronic properties that enhance selectivity in oxidation reactions, making them efficient in partial oxidation processes. Palladium catalysts demonstrate superior efficiency in hydrogenation reactions due to their high surface area and ability to activate hydrogen molecules effectively. Comparative studies reveal that while palladium offers higher catalytic turnover frequencies, antimony provides greater selectivity towards desired products, optimizing reaction pathways in complex organic syntheses.
Cost and Availability Comparison
Antimony is significantly more abundant and less expensive than palladium, making it a cost-effective choice for catalytic applications. Palladium, a rare precious metal predominantly sourced from mining regions like Russia and South Africa, commands higher prices due to limited availability and increasing demand in automotive catalytic converters. The cost disparity and global supply constraints often influence the selection of antimony over palladium in industrial catalysis where budget and material accessibility are critical factors.
Environmental Impact and Toxicity
Antimony and palladium exhibit distinct environmental and toxicity profiles when used as catalysts; antimony poses significant ecological risks due to its persistence, bioaccumulation, and potential carcinogenic effects on aquatic life and humans. Palladium, although generally considered less toxic, can still cause environmental contamination through mining and industrial processes, but it tends to have a lower bioaccumulation potential and biodegradability impact. The selection of palladium over antimony in catalytic applications often aligns with efforts to reduce hazardous waste and minimize long-term ecological damage.
Industrial Applications and Case Studies
Antimony exhibits strong oxidative and dehydrogenation catalytic properties, making it valuable in industrial applications such as flame retardants and semiconductors, whereas palladium is prominent for hydrogenation, carbon-carbon coupling reactions, and automotive catalytic converters. Case studies highlight palladium's superior efficiency in catalytic converters reducing NOx emissions, while antimony-based catalysts demonstrate effective use in polymerization and plastic production processes. The choice between antimony and palladium catalysts depends on specific industrial reaction requirements, cost factors, and target product selectivity.
Future Trends in Catalyst Development
Antimony shows promise in catalyst development due to its low cost and ability to enhance selectivity in hydrogenation reactions, while palladium remains a benchmark for high catalytic activity in fine chemical synthesis. Future trends indicate a growing emphasis on antimony-based catalysts integrated with nanostructured supports to improve durability and reduce palladium dependency. Research is focusing on hybrid catalysts combining antimony and palladium to achieve cost-effective performance with improved environmental sustainability in automotive and industrial catalytic applications.
Conclusion: Choosing Between Antimony and Palladium
Antimony and palladium serve distinct roles as catalysts, with palladium excelling in hydrogenation and carbon-carbon coupling reactions due to its superior activity and selectivity. Antimony, while less active, offers advantages in cost-effectiveness and unique catalytic properties for specific oxidation reactions. Selecting between antimony and palladium catalysts depends on balancing economic factors, desired reaction pathways, and compatibility with reaction conditions to optimize performance.
Infographic: Antimony vs Palladium for Catalyst
 azmater.com
azmater.com