Gallium plating offers superior corrosion resistance and lower toxicity compared to chromium plating, making it ideal for eco-friendly applications. Chromium plating provides exceptional hardness and wear resistance, preferred in automotive and industrial components requiring durability.
Table of Comparison
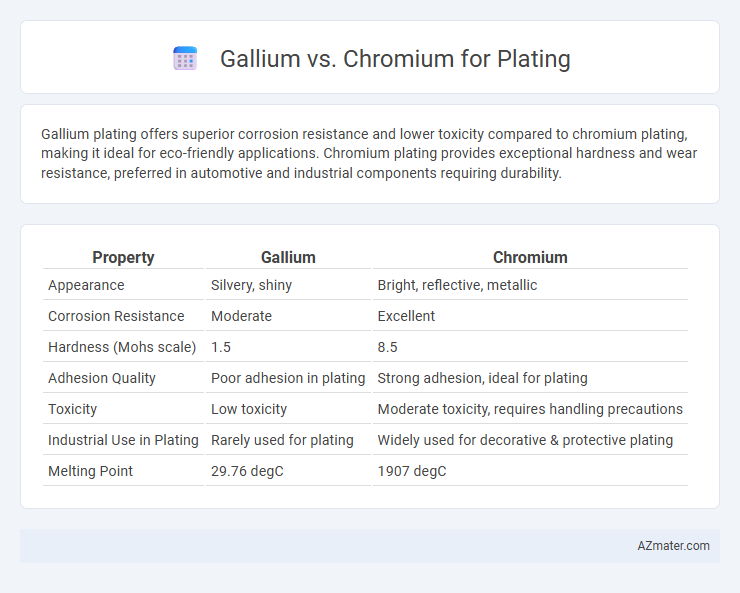
| Property | Gallium | Chromium |
|---|---|---|
| Appearance | Silvery, shiny | Bright, reflective, metallic |
| Corrosion Resistance | Moderate | Excellent |
| Hardness (Mohs scale) | 1.5 | 8.5 |
| Adhesion Quality | Poor adhesion in plating | Strong adhesion, ideal for plating |
| Toxicity | Low toxicity | Moderate toxicity, requires handling precautions |
| Industrial Use in Plating | Rarely used for plating | Widely used for decorative & protective plating |
| Melting Point | 29.76 degC | 1907 degC |
Introduction to Plating: Gallium vs Chromium
Gallium and chromium are both used in plating processes but serve distinct purposes due to their unique properties. Chromium plating is renowned for its hardness, corrosion resistance, and shiny finish, making it ideal for automotive, industrial, and decorative applications. Gallium plating, though less common, offers excellent wettability and low toxicity, often favored in electronic components and specialty coatings for improved conductivity and flexibility.
Chemical Properties Comparison
Gallium exhibits a low melting point of about 29.76degC and demonstrates poor hardness, making it less suitable for durable plating compared to chromium, which has a high melting point of 1907degC and exceptional hardness that provides excellent wear resistance. Chemically, gallium is more reactive, forming oxides rapidly in air, while chromium forms a stable, protective oxide layer that enhances corrosion resistance. Chromium's superior chemical stability and hardness make it the preferred choice for plating applications requiring durability and protection against oxidation.
Electroplating Process Differences
Gallium and chromium exhibit distinct differences in electroplating processes due to their unique electrochemical properties. Chromium plating typically involves hexavalent chromium in acidic baths with high current densities, producing hard, corrosion-resistant coatings, whereas gallium plating requires specialized aqueous solutions and lower current densities for uniform deposition. The deposition potential, bath composition, and resulting coating morphology significantly impact the plating quality and application suitability for each metal.
Durability and Corrosion Resistance
Gallium plating offers moderate durability with limited corrosion resistance, primarily used for specialized applications requiring low melting points and unique electrical properties. Chromium plating excels in durability and corrosion resistance, forming a hard, protective layer that withstands wear, oxidation, and harsh environmental conditions commonly found in automotive and industrial parts. The superior hardness and chemical stability of chromium make it the preferred choice for long-lasting, corrosion-resistant coatings over gallium.
Surface Finish and Aesthetics
Gallium plating offers a smooth, highly reflective surface finish that enhances aesthetic appeal with a silvery luster, making it suitable for decorative applications. Chromium plating provides a durable, corrosion-resistant coating with a bright, mirror-like finish favored in automotive and hardware industries for its long-lasting visual impact. While gallium excels in achieving a unique, lustrous sheen, chromium is preferred for toughness and consistent surface quality in high-wear environments.
Industrial Applications and Uses
Gallium and chromium serve distinct roles in industrial plating, with chromium widely utilized for its hardness, corrosion resistance, and aesthetic appeal in automotive, aerospace, and tool manufacturing. Gallium plating, though less common, finds niche applications in electronics and semiconductor industries due to its excellent thermal and electrical conductivity. Chromium's robust durability and wear resistance make it the preferred choice for heavy-duty industrial parts, whereas gallium's unique properties are leveraged in advanced technological coatings.
Environmental Impact and Safety
Gallium plating demonstrates lower environmental toxicity compared to chromium, reducing hazardous waste generation and minimizing soil and water contamination risks. Chromium plating, especially hexavalent chromium, poses significant health hazards due to its carcinogenic and mutagenic properties, necessitating strict safety protocols and protective equipment. Gallium offers a safer alternative with less stringent handling requirements, making it preferable for eco-friendly and worker-safe industrial applications.
Cost and Material Availability
Gallium plating is significantly more expensive than chromium due to the rarity and high extraction costs of gallium, which is primarily obtained as a byproduct of aluminum and zinc production. Chromium plating benefits from widespread availability and established mining infrastructure, making it a more cost-effective choice for large-scale industrial applications. Material availability heavily favors chromium, as it is more abundant and easier to source, ensuring lower prices and steady supply chains compared to the limited and specialized gallium deposits.
Challenges and Limitations
Gallium plating faces challenges such as poor adhesion to substrates and low hardness, limiting its use in wear-resistant applications compared to chromium, which offers superior hardness and corrosion resistance. Chromium plating excels in durability but involves toxic hexavalent chromium emissions, creating environmental and health limitations. Both metals require precise process controls to optimize plating quality and mitigate operational challenges in industrial settings.
Future Prospects in Plating Technologies
Gallium presents promising future prospects in plating technologies due to its low toxicity and excellent adhesion properties, making it a sustainable alternative to traditional chromium plating. While chromium offers superior hardness and corrosion resistance, growing environmental regulations and health concerns restrict its widespread usage, driving research towards gallium-based coatings. Emerging innovations in gallium plating aim to enhance wear resistance and electrical conductivity, positioning it as a viable candidate for next-generation protective and decorative surface treatments.
Infographic: Gallium vs Chromium for Plating
 azmater.com
azmater.com