Gallium alloys offer superior thermal conductivity and low melting points compared to zinc alloys, enhancing their performance in electronics and heat transfer applications. Zinc alloys provide greater mechanical strength and corrosion resistance, making them ideal for structural and automotive components.
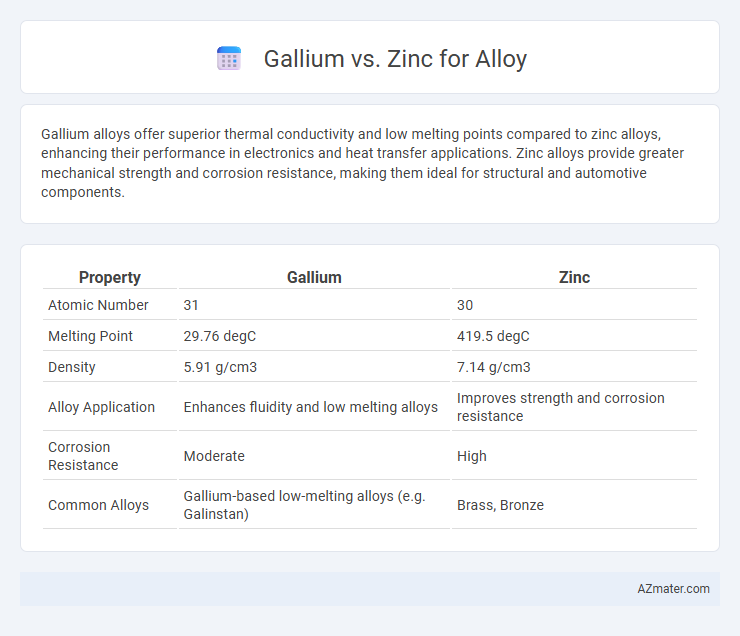
Table of Comparison
| Property | Gallium | Zinc |
|---|---|---|
| Atomic Number | 31 | 30 |
| Melting Point | 29.76 degC | 419.5 degC |
| Density | 5.91 g/cm3 | 7.14 g/cm3 |
| Alloy Application | Enhances fluidity and low melting alloys | Improves strength and corrosion resistance |
| Corrosion Resistance | Moderate | High |
| Common Alloys | Gallium-based low-melting alloys (e.g. Galinstan) | Brass, Bronze |
Introduction to Gallium and Zinc in Alloys
Gallium and zinc are essential metals widely used in alloy formulations for enhanced mechanical and thermal properties. Gallium, known for its low melting point and ability to form eutectic alloys, improves fluidity and corrosion resistance in metal mixtures. Zinc, valued for its strength and anti-corrosion characteristics, is commonly alloyed with copper and aluminum to produce durable materials used in automotive and construction industries.
Chemical and Physical Properties Comparison
Gallium exhibits a low melting point of 29.76degC and high malleability, making it ideal for low-temperature alloys, while zinc melts at 419.5degC and provides greater hardness and corrosion resistance in alloys. Chemically, gallium is less reactive than zinc, forming stable oxides and exhibiting amphoteric behavior, whereas zinc typically shows greater reactivity with acids and bases. The differences in atomic structure--gallium's orthorhombic crystal system versus zinc's hexagonal close-packed lattice--impact their alloying behavior, with zinc enhancing strength and gallium improving ductility and thermal conductivity.
Melting Points and Thermal Behavior
Gallium exhibits a low melting point of about 29.8degC, enabling alloys to become liquid near room temperature, while zinc melts at a much higher temperature of 419.5degC, contributing to solid and durable alloy formations. Gallium-based alloys display excellent thermal expansion properties and good heat dissipation, making them suitable for applications requiring precise thermal management. Zinc alloys offer higher thermal stability and resistance to corrosion, ideal for structural components subjected to elevated temperatures.
Alloying Capabilities and Compatibilities
Gallium exhibits exceptional alloying capabilities due to its low melting point and ability to wet metals like aluminum, enabling the formation of strong, corrosion-resistant alloys often used in electronics and thermometers. Zinc, with its higher melting point and excellent compatibility with copper, enhances mechanical strength and corrosion resistance in brass and other copper alloys commonly utilized in construction and marine applications. Both elements contribute distinct properties to alloys, with gallium favoring low-temperature, flexible applications and zinc excelling in structural, high-strength metal composites.
Mechanical Strength and Hardness Differences
Gallium alloys typically exhibit lower mechanical strength compared to zinc alloys due to gallium's low melting point and softness, which reduce hardness and load-bearing capacity. Zinc alloys possess higher hardness and tensile strength, making them more suitable for structural and industrial applications requiring durability. The inclusion of zinc improves wear resistance and rigidity, while gallium enhances malleability and corrosion resistance but compromises overall mechanical robustness.
Corrosion Resistance: Gallium vs Zinc
Gallium alloys exhibit superior corrosion resistance compared to zinc alloys due to their ability to form stable oxide layers that protect the metal surface from further degradation. Zinc alloys, while offering fair corrosion resistance, are more prone to galvanic corrosion and require additional protective coatings in harsh environments. This makes gallium-based alloys preferable for applications demanding long-term durability in corrosive conditions.
Industrial and Technological Applications
Gallium and zinc alloys serve distinct roles in industrial and technological applications due to their differing melting points and mechanical properties. Gallium, with its low melting point near 29.8degC, facilitates the creation of low-temperature fusible alloys used in thermal switches and heat transfer systems, while zinc alloys offer high strength and corrosion resistance ideal for die casting in automotive and electronics manufacturing. The integration of gallium enhances soldering and semiconductor technologies, whereas zinc's versatility supports structural components and protective coatings in various engineering sectors.
Environmental Impact and Safety Concerns
Gallium alloys exhibit low toxicity and minimal environmental impact due to their stable chemical properties and limited bioaccumulation, making them safer for industrial and medical applications. Zinc alloys, while widely used, pose moderate environmental risks through soil and water contamination from mining and manufacturing processes, contributing to ecological toxicity and bioavailability issues. Gallium's non-reactive nature reduces safety hazards during handling, whereas zinc's potential for dust and fume generation requires stricter safety protocols to prevent respiratory and dermal exposure.
Cost and Availability for Alloy Production
Gallium alloys tend to be more expensive than zinc alloys due to gallium's relative scarcity and higher extraction costs, with gallium priced around $300 per kilogram compared to zinc's average price of $2 to $3 per kilogram. Zinc's abundant availability and well-established mining infrastructure make it a cost-effective choice for large-scale alloy production. Manufacturers often prefer zinc alloys for economic efficiency and supply stability in industrial applications.
Future Prospects and Innovations in Alloying
Gallium's low melting point and ability to improve metal malleability position it as a promising element for next-generation alloys in flexible electronics and advanced thermal management systems. Zinc offers cost-effective corrosion resistance and excellent strength-to-weight ratios, driving innovations in sustainable construction materials and automotive components. Future research aims to combine gallium's unique physicochemical properties with zinc's durability, potentially creating hybrid alloys with enhanced performance for aerospace and energy applications.
Infographic: Gallium vs Zinc for Alloy
 azmater.com
azmater.com