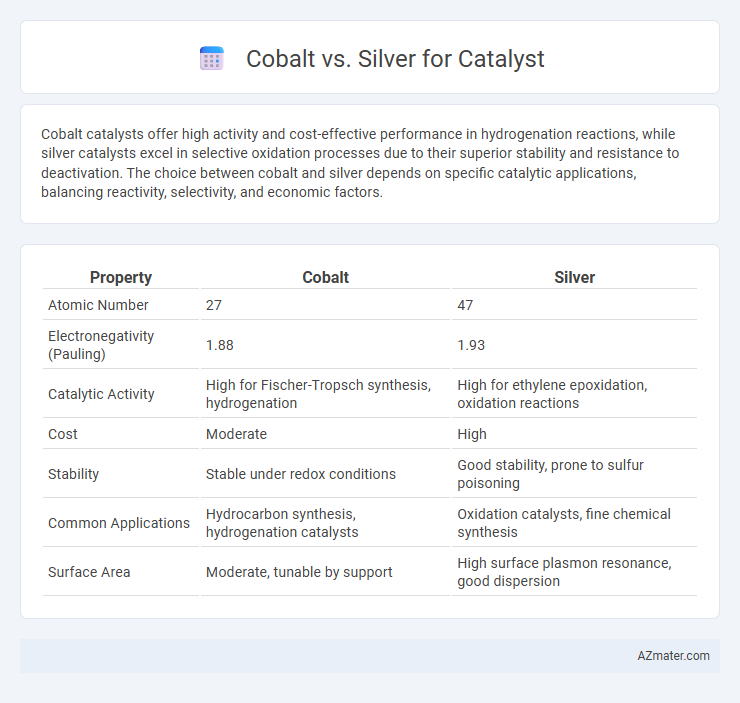
Cobalt catalysts offer high activity and cost-effective performance in hydrogenation reactions, while silver catalysts excel in selective oxidation processes due to their superior stability and resistance to deactivation. The choice between cobalt and silver depends on specific catalytic applications, balancing reactivity, selectivity, and economic factors.
Table of Comparison
| Property | Cobalt | Silver |
|---|---|---|
| Atomic Number | 27 | 47 |
| Electronegativity (Pauling) | 1.88 | 1.93 |
| Catalytic Activity | High for Fischer-Tropsch synthesis, hydrogenation | High for ethylene epoxidation, oxidation reactions |
| Cost | Moderate | High |
| Stability | Stable under redox conditions | Good stability, prone to sulfur poisoning |
| Common Applications | Hydrocarbon synthesis, hydrogenation catalysts | Oxidation catalysts, fine chemical synthesis |
| Surface Area | Moderate, tunable by support | High surface plasmon resonance, good dispersion |
Introduction to Cobalt and Silver as Catalysts
Cobalt and silver are widely used transition metals in catalysis, each exhibiting distinct chemical properties that influence their catalytic performance. Cobalt serves as an efficient catalyst in processes like Fischer-Tropsch synthesis and hydrogenation reactions due to its ability to facilitate electron transfer and form stable intermediates. Silver catalysts are prominent in selective oxidation reactions and ethylene epoxidation, benefiting from silver's unique surface chemistry and strong affinity for oxygen species.
Chemical Properties Influencing Catalytic Activity
Cobalt exhibits variable oxidation states, primarily +2 and +3, enabling versatile redox reactions that enhance catalytic activity in processes like Fischer-Tropsch synthesis. Silver, with its stable +1 oxidation state and weaker oxophilicity, favors selective oxidation reactions due to its higher electron density and surface plasmon resonance effects. The differing electronic configurations and bonding energies of cobalt and silver crucially influence their catalytic efficiency, selectivity, and reaction pathways in heterogeneous catalysis.
Cost and Availability of Cobalt vs Silver
Cobalt offers a more cost-effective alternative to silver as a catalyst due to its significantly lower market price and greater abundance in the Earth's crust. While silver is highly effective, its high cost and limited availability constrain large-scale industrial applications, making cobalt a preferred choice in cost-sensitive catalytic processes. The widespread mining of cobalt from countries such as the Democratic Republic of Congo ensures a more stable supply chain compared to the relatively scarce silver deposits.
Applications in Industrial Catalysis
Cobalt catalysts exhibit superior performance in Fischer-Tropsch synthesis and hydrodesulfurization processes due to their high activity and selectivity for long-chain hydrocarbons, making them indispensable in refining and petrochemical industries. Silver catalysts, valued for their ability to facilitate ethylene epoxidation and selective oxidation reactions, are widely applied in the production of ethylene oxide and formaldehyde, critical intermediates in chemical manufacturing. Industrial catalysis leverages cobalt's robustness in hydrogenation and cobalt-based catalysts' resistance to poisoning, while silver's catalytic properties excel in low-temperature oxidation, optimizing efficiency in large-scale chemical production.
Environmental Impact and Sustainability
Cobalt catalysts often raise environmental concerns due to toxic mining practices and limited recyclability, leading to soil and water contamination. Silver catalysts offer improved sustainability by enabling lower reaction temperatures and higher efficiency, reducing energy consumption and carbon footprint. However, silver's rarity necessitates responsible sourcing and recycling initiatives to mitigate supply chain environmental impacts.
Catalytic Performance and Efficiency Comparison
Cobalt catalysts demonstrate superior catalytic performance with higher turnover frequencies and greater selectivity in processes such as Fischer-Tropsch synthesis compared to silver catalysts. Silver exhibits excellent activity in oxidation reactions but often lags behind cobalt in terms of efficiency and durability under harsh reaction conditions. The enhanced electronic properties and resistance to sintering give cobalt catalysts a significant advantage in industrial applications demanding sustained catalytic efficiency.
Stability and Longevity in Catalytic Processes
Cobalt catalysts exhibit superior stability and longevity in high-temperature and oxidative catalytic processes compared to silver, due to their robust resistance to sintering and oxidation. Silver catalysts, while highly active in reactions like ethylene epoxidation, tend to suffer from rapid deactivation and lower thermal durability under rigorous conditions. The choice between cobalt and silver catalysts hinges on the specific process demands, with cobalt favored for sustained catalytic activity and silver preferred for selectivity in milder environments.
Recent Advances in Catalyst Design
Recent advances in catalyst design reveal cobalt as a cost-effective alternative to silver, showing high catalytic activity in oxygen evolution reactions (OER) and carbon dioxide reduction. Cobalt-based catalysts exhibit enhanced stability and tunable electronic properties through nanostructuring and doping techniques, which improve catalytic efficiency comparable to silver. Silver remains superior in selective CO2 reduction to ethylene, but innovations in cobalt catalyst design are closing the performance gap while offering scalability and sustainability benefits.
Challenges in Using Cobalt and Silver Catalysts
Cobalt catalysts often face challenges such as poor selectivity and rapid deactivation due to sintering and oxidation under reaction conditions, limiting their long-term stability in industrial applications. Silver catalysts, while exhibiting excellent selectivity for certain reactions like ethylene epoxidation, suffer from low activity and vulnerability to surface poisoning by sulfur and chlorine compounds. Both metals require advanced support materials and precise control over particle size and dispersion to overcome these limitations and enhance catalytic performance.
Future Trends in Catalyst Development
Cobalt-based catalysts are gaining momentum due to their superior activity and cost-effectiveness compared to traditional silver catalysts, especially in sustainable energy applications like hydrogen production and CO2 reduction. Advances in nanostructuring and doping techniques further enhance cobalt catalysts' selectivity and durability, promising significant improvements in industrial catalytic processes. The shift toward earth-abundant metals like cobalt aligns with growing environmental regulations and the demand for scalable, eco-friendly catalyst materials in future innovations.
Infographic: Cobalt vs Silver for Catalyst
 azmater.com
azmater.com