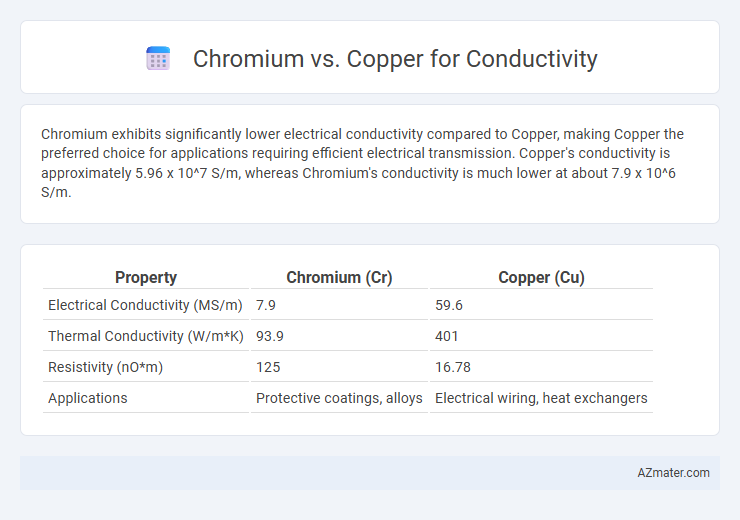
Chromium exhibits significantly lower electrical conductivity compared to Copper, making Copper the preferred choice for applications requiring efficient electrical transmission. Copper's conductivity is approximately 5.96 x 10^7 S/m, whereas Chromium's conductivity is much lower at about 7.9 x 10^6 S/m.
Table of Comparison
| Property | Chromium (Cr) | Copper (Cu) |
|---|---|---|
| Electrical Conductivity (MS/m) | 7.9 | 59.6 |
| Thermal Conductivity (W/m*K) | 93.9 | 401 |
| Resistivity (nO*m) | 125 | 16.78 |
| Applications | Protective coatings, alloys | Electrical wiring, heat exchangers |
Introduction to Electrical Conductivity
Electrical conductivity measures a material's ability to allow the flow of electric current, primarily influenced by the density and mobility of free electrons. Copper exhibits high electrical conductivity at approximately 5.96 x 10^7 S/m due to its single 4s electron and minimal electron scattering. Chromium, with a conductivity around 7.9 x 10^6 S/m, has more complex electron interactions and a higher resistivity influenced by its 3d electron configuration, making it less efficient than copper for conducting electricity.
Overview of Chromium and Copper
Copper exhibits superior electrical conductivity, measured at approximately 5.96 x 10^7 siemens per meter, making it the industry standard for electrical wiring and components. Chromium, while offering excellent corrosion resistance and hardness, has significantly lower conductivity, around 7.9 x 10^6 siemens per meter, limiting its use in electrical applications. The distinct properties of copper and chromium influence their selection: copper excels in high-conductivity requirements, whereas chromium is favored for protective coatings and wear resistance.
Atomic Structure and Conductivity
Chromium's atomic structure features a body-centered cubic lattice with 24 electrons, resulting in lower electron mobility and reduced conductivity compared to copper, which has a face-centered cubic lattice and a single valence electron enabling high electron mobility. Copper's atomic arrangement facilitates efficient free electron flow, making it one of the best conductors of electricity with a conductivity of approximately 5.96 x 10^7 S/m. The partially filled 3d subshell in chromium causes increased electron scattering, significantly diminishing its electrical conductivity relative to copper.
Electrical Conductivity Ratings: Chromium vs Copper
Copper boasts an electrical conductivity rating of approximately 59.6 x 10^6 S/m, making it one of the most efficient conductors used in electrical wiring and components. Chromium significantly lags behind, with an electrical conductivity near 7.9 x 10^6 S/m, roughly 13% that of copper, limiting its use in applications where high conductivity is critical. The stark contrast in conductivity ratings underscores copper's dominance in electrical and electronic industries, while chromium is favored primarily for corrosion resistance and hardness rather than electrical performance.
Thermal Conductivity Comparison
Copper exhibits significantly higher thermal conductivity than chromium, with copper averaging around 401 W/m*K compared to chromium's approximately 93.9 W/m*K at room temperature. This substantial difference makes copper the preferred material for applications requiring efficient heat dissipation and thermal management. Chromium's lower thermal conductivity limits its use in heat-sensitive environments despite its excellent corrosion resistance and hardness.
Corrosion Resistance and Longevity
Copper exhibits superior conductivity compared to chromium, making it a preferred choice in electrical applications. Chromium excels in corrosion resistance, forming a durable oxide layer that protects metals from rust and degradation over time. The longevity of copper can be compromised by corrosion in harsh environments, whereas chromium's corrosion-resistant properties significantly enhance the lifespan of coatings and alloys.
Practical Applications in Industry
Chromium offers moderate electrical conductivity, making it suitable for corrosion-resistant coatings and plating in electronics, while copper excels with one of the highest electrical conductivity values, ideal for electrical wiring, motors, and circuit boards. Copper's superior conductivity and ductility make it the preferred choice in power transmission and electrical components, whereas chromium is often used to enhance surface durability without significantly improving conductivity. Industrial applications prioritize copper for efficient current flow, with chromium's role focused more on protective layering rather than direct electrical performance.
Cost and Availability
Copper outperforms chromium in electrical conductivity, making it the preferred choice for most conductive applications. Copper's widespread availability and relatively low cost support its extensive use in electrical wiring and electronics, whereas chromium is more expensive and less abundant, limiting its practicality despite its corrosion resistance. Cost-effectiveness and accessibility position copper as the dominant material in conductive industries compared to chromium.
Environmental Impact
Chromium and copper differ significantly in environmental impact related to conductivity applications; copper exhibits superior electrical conductivity at 5.96 x 10^7 S/m, enabling efficient energy transmission and reducing power loss, which helps lower carbon emissions from electricity generation. Chromium, though less conductive with approximately 7.9 x 10^6 S/m, is primarily valued for its corrosion resistance rather than conductivity, but its extraction and processing involve toxic chromium compounds posing environmental and health risks. Copper mining and recycling have established more environmentally sustainable practices, making copper the preferred choice for conductive materials to minimize ecological footprint.
Conclusion: Choosing the Right Metal
Chromium offers moderate electrical conductivity with superior corrosion resistance, making it suitable for specialized applications requiring durability. Copper exhibits high electrical conductivity, around 5.96 x 10^7 S/m, making it ideal for efficient electrical wiring and components. Selecting between chromium and copper depends on prioritizing either conductivity performance or environmental stability in the intended use.
Infographic: Chromium vs Copper for Conductivity
 azmater.com
azmater.com