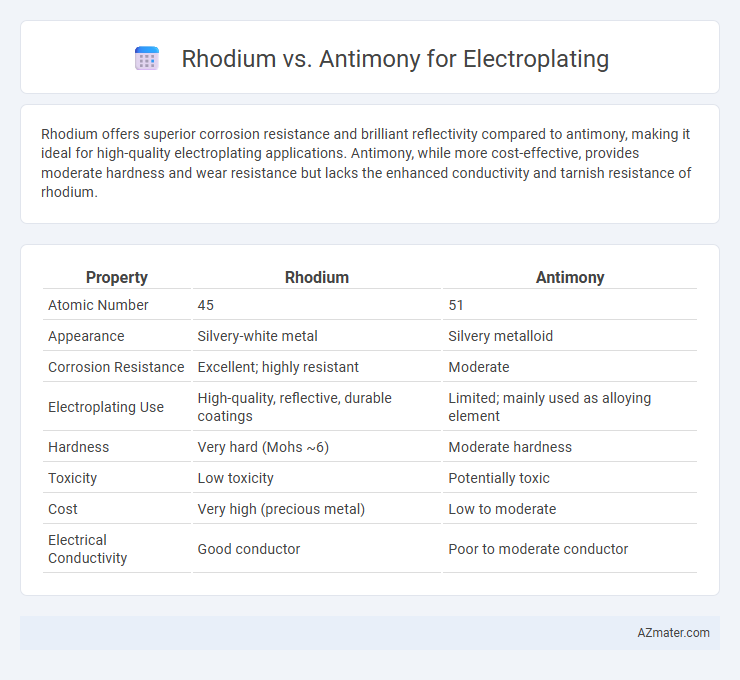
Rhodium offers superior corrosion resistance and brilliant reflectivity compared to antimony, making it ideal for high-quality electroplating applications. Antimony, while more cost-effective, provides moderate hardness and wear resistance but lacks the enhanced conductivity and tarnish resistance of rhodium.
Table of Comparison
| Property | Rhodium | Antimony |
|---|---|---|
| Atomic Number | 45 | 51 |
| Appearance | Silvery-white metal | Silvery metalloid |
| Corrosion Resistance | Excellent; highly resistant | Moderate |
| Electroplating Use | High-quality, reflective, durable coatings | Limited; mainly used as alloying element |
| Hardness | Very hard (Mohs ~6) | Moderate hardness |
| Toxicity | Low toxicity | Potentially toxic |
| Cost | Very high (precious metal) | Low to moderate |
| Electrical Conductivity | Good conductor | Poor to moderate conductor |
Introduction to Electroplating Materials
Rhodium and antimony serve distinct roles in electroplating due to their unique chemical and physical properties. Rhodium, a rare and corrosion-resistant noble metal, is prized for its brilliant, reflective finish and exceptional hardness, making it ideal for decorative and protective coatings on jewelry and automotive parts. Antimony, often used as an alloying element, enhances hardness and wear resistance in electroplated layers but is less common as a standalone plating material compared to rhodium.
Overview of Rhodium and Antimony
Rhodium is a rare, precious metal known for its exceptional corrosion resistance, high reflectivity, and hardness, making it ideal for electroplating applications requiring durable, bright, and tarnish-resistant coatings. Antimony, a metalloid, is primarily used as an alloying element in plating baths to improve the mechanical properties and brightness of deposits, though it is less common as a standalone plating metal. Rhodium electroplating offers superior chemical stability and a brilliant finish, while antimony enhances hardness and wear resistance when included in plating formulations.
Chemical Properties Comparison
Rhodium exhibits exceptional corrosion resistance and a high melting point around 1964degC, making it ideal for durable electroplating applications, while antimony has a considerably lower melting point of 630.6degC and moderate chemical stability. Rhodium's inertness to acids and excellent electrical conductivity enhance its suitability for protective and decorative coatings, whereas antimony often serves as a hardening additive but lacks rhodium's robustness. The significant difference in oxidation states, with rhodium commonly in +3 and +4 states compared to antimony's +3 and +5, influences their plating characteristics and chemical behavior in electrochemical solutions.
Electroplating Efficiency and Performance
Rhodium offers exceptional electroplating efficiency due to its high corrosion resistance and excellent conductivity, resulting in a bright, durable finish ideal for jewelry and electronics. Antimony, while less conductive, enhances hardness and wear resistance when alloyed in electroplated coatings, improving overall performance in industrial applications. Rhodium's superior plating uniformity and minimal surface defects outperform antimony, making it the preferred choice for high-precision electroplating processes.
Surface Finish and Aesthetic Qualities
Rhodium electroplating delivers a highly reflective, mirror-like surface finish with exceptional brightness and corrosion resistance, making it ideal for jewelry and decorative applications. Antimony plating, while providing a duller, matte finish, enhances surface hardness and offers good wear resistance but lacks the lustrous aesthetic appeal of rhodium. The choice between rhodium and antimony for electroplating depends on the desired balance between aesthetic brilliance and functional durability.
Durability and Corrosion Resistance
Rhodium excels in electroplating due to its exceptional durability and superior corrosion resistance, making it ideal for applications requiring long-lasting, tarnish-free coatings. Antimony, while offering moderate corrosion resistance, is less durable and prone to oxidation compared to rhodium, limiting its effectiveness in harsh environments. The high hardness and chemical inertness of rhodium provide a more robust protective layer against wear and environmental damage.
Cost Analysis: Rhodium vs Antimony
Rhodium electroplating commands a significantly higher cost compared to antimony due to its rarity and superior corrosion resistance, often priced at over $10,000 per ounce versus antimony's average market price below $10 per pound. The expense of rhodium plating is justified in high-end applications like automotive trim and jewelry, where its brilliant finish and durability add premium value. Antimony plating serves more cost-sensitive industrial uses, providing adequate protection and conductivity at a fraction of rhodium's cost, making it suitable for mass production and less decorative purposes.
Environmental and Safety Considerations
Rhodium electroplating offers superior corrosion resistance with lower environmental toxicity compared to antimony, which involves the use of more hazardous chemicals and generates toxic waste. The disposal of antimony plating bath residues poses significant ecological risks due to its heavy metal content, requiring strict hazardous waste management protocols. Rhodium plating solutions require careful handling but exhibit reduced environmental impact and improved worker safety profiles in industrial applications.
Industrial Applications and Use Cases
Rhodium offers superior corrosion resistance, high reflectivity, and excellent hardness, making it ideal for automotive catalytic converters, jewelry plating, and electrical contacts where durability and aesthetic appeal are critical. Antimony is primarily used in electroplating to enhance the wear resistance and hardness of lead and tin alloys, benefiting applications such as battery grids, bearings, and ammunition coatings. Industrially, rhodium's high cost limits its use to premium products, while antimony's affordability and alloying properties make it suitable for heavy-duty and cost-sensitive electroplating scenarios.
Conclusion: Choosing the Right Electroplating Material
Rhodium offers superior corrosion resistance, brilliant luster, and excellent hardness, making it ideal for high-end jewelry and electronic contacts. Antimony provides cost-effective wear resistance and improved solderability but lacks the exceptional shine and durability of rhodium. Selecting the right electroplating material depends on balancing budget constraints with the desired finish quality and corrosion protection.
Infographic: Rhodium vs Antimony for Electroplating
 azmater.com
azmater.com