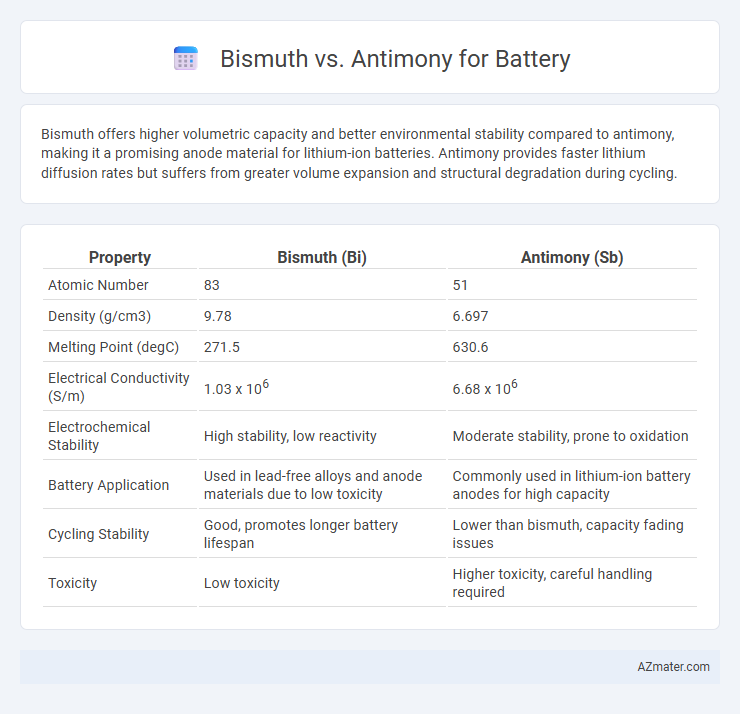
Bismuth offers higher volumetric capacity and better environmental stability compared to antimony, making it a promising anode material for lithium-ion batteries. Antimony provides faster lithium diffusion rates but suffers from greater volume expansion and structural degradation during cycling.
Table of Comparison
| Property | Bismuth (Bi) | Antimony (Sb) |
|---|---|---|
| Atomic Number | 83 | 51 |
| Density (g/cm3) | 9.78 | 6.697 |
| Melting Point (degC) | 271.5 | 630.6 |
| Electrical Conductivity (S/m) | 1.03 x 106 | 6.68 x 106 |
| Electrochemical Stability | High stability, low reactivity | Moderate stability, prone to oxidation |
| Battery Application | Used in lead-free alloys and anode materials due to low toxicity | Commonly used in lithium-ion battery anodes for high capacity |
| Cycling Stability | Good, promotes longer battery lifespan | Lower than bismuth, capacity fading issues |
| Toxicity | Low toxicity | Higher toxicity, careful handling required |
Introduction to Bismuth and Antimony in Battery Technology
Bismuth and antimony are emerging as crucial materials in battery technology due to their high theoretical capacities and favorable electrochemical properties. Bismuth offers low toxicity and excellent cycling stability, making it suitable for stable anode performance in lithium-ion and sodium-ion batteries. Antimony exhibits higher capacity but faces challenges with volume expansion, prompting research into alloying and nanostructuring to improve battery lifespan and efficiency.
Chemical and Physical Properties Comparison
Bismuth and antimony are both metalloids with significant differences influencing their battery applications; bismuth has a lower melting point (271.4degC) and higher density (9.78 g/cm3) compared to antimony's melting point of 630.6degC and density of 6.68 g/cm3. Chemically, bismuth exhibits greater corrosion resistance and forms stable Bi3+ ions, benefiting electrode longevity, whereas antimony, with its ability to alloy easily and variable oxidation states (Sb3+ and Sb5+), enhances capacity but may suffer from volume expansion during cycling. The higher electrical conductivity and mechanical stability of antimony improve charge transport but increase structural degradation risk, while bismuth's structural robustness and low toxicity make it favorable for safer, longer-life battery designs.
Abundance and Sustainability of Bismuth vs Antimony
Bismuth is more abundant in the Earth's crust compared to antimony, making it a more sustainable choice for battery applications due to its wider availability and lower geopolitical sourcing risks. While antimony is less abundant and often associated with environmental and health concerns during mining, bismuth offers a non-toxic alternative with less environmental impact. The sustainability of bismuth batteries is further supported by its recyclability and reduced reliance on critical raw materials, enhancing long-term resource security.
Electrochemical Performance in Battery Applications
Bismuth demonstrates superior electrochemical performance compared to antimony in battery applications due to its higher volumetric capacity and better cycling stability, which results in prolonged battery lifespan. Bismuth's lower alloying/dealloying voltage hysteresis enhances energy efficiency and reduces capacity fading during charge-discharge cycles. In contrast, antimony faces challenges with structural degradation and volume expansion, leading to reduced cyclic stability and lower overall capacity retention.
Toxicity and Environmental Impact
Bismuth exhibits significantly lower toxicity compared to antimony, making it a safer choice for battery applications from a health perspective. Environmental impact studies reveal that bismuth is more environmentally benign, with minimal leaching and bioaccumulation risks, unlike antimony, which poses higher ecological hazards due to its persistence and toxicity in soil and water. Batteries incorporating bismuth therefore offer improved safety and sustainability profiles over those using antimony-based materials.
Cost and Market Availability
Bismuth offers a moderate cost advantage over antimony due to its more abundant global reserves and lower extraction expenses, making it increasingly attractive for battery applications. Antimony's market availability is constrained by geopolitical factors and limited mining regions, driving up its price volatility in battery manufacturing. The cost-efficiency and steady supply of bismuth enable more stable production of advanced energy storage devices compared to antimony-based systems.
Battery Types Benefiting from Bismuth
Bismuth enhances the performance of lead-acid and sodium-ion batteries by improving cycle life and capacity retention due to its high density and low toxicity. Its excellent alloying properties with lead increase battery durability and reduce corrosion in lead-acid systems. In contrast, antimony, while effective in lead-acid battery grids for conductivity, presents higher toxicity, making bismuth a safer alternative for advanced battery chemistries.
Battery Types Benefiting from Antimony
Antimony enhances lead-acid battery performance by improving charge acceptance and cycle life, particularly in deep-cycle and starting batteries used in automotive and industrial applications. Its alloying with lead increases mechanical strength and corrosion resistance, crucial for starting, lighting, and ignition (SLI) batteries. Batteries like valve-regulated lead-acid (VRLA) and absorbed glass mat (AGM) benefit significantly from antimony's ability to improve efficiency and longevity under high load conditions.
Emerging Research and Innovations
Bismuth-based electrodes exhibit exceptional stability and high capacity in battery applications, with emerging research highlighting their potential to replace heavy metals like lead and mercury in energy storage. Antimony anodes demonstrate remarkable theoretical capacity and fast lithium-ion diffusion, driving innovations in alloying strategies and nanostructuring to enhance cyclic stability and rate performance. Recent studies emphasize combining bismuth and antimony in composite materials to leverage synergistic effects, leading to improved conductivity and extended battery lifespan in next-generation rechargeable batteries.
Future Outlook and Industry Recommendations
Bismuth and antimony both offer promising future applications in battery technology due to their high theoretical capacities and improved safety profiles compared to traditional materials. Industry trends indicate a growing preference for bismuth in next-generation anodes because of its lower toxicity and better cycling stability, while antimony's higher electrical conductivity makes it suitable for hybrid battery systems. Experts recommend continued research on alloying bismuth with other elements and optimizing antimony's nanostructures to enhance energy density and lifespan in commercial battery applications.
Infographic: Bismuth vs Antimony for Battery
 azmater.com
azmater.com