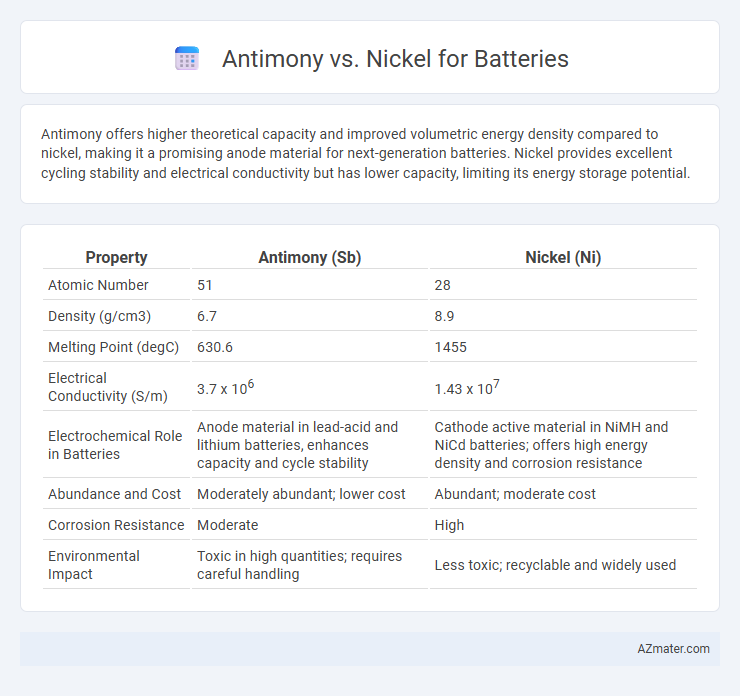
Antimony offers higher theoretical capacity and improved volumetric energy density compared to nickel, making it a promising anode material for next-generation batteries. Nickel provides excellent cycling stability and electrical conductivity but has lower capacity, limiting its energy storage potential.
Table of Comparison
| Property | Antimony (Sb) | Nickel (Ni) |
|---|---|---|
| Atomic Number | 51 | 28 |
| Density (g/cm3) | 6.7 | 8.9 |
| Melting Point (degC) | 630.6 | 1455 |
| Electrical Conductivity (S/m) | 3.7 x 106 | 1.43 x 107 |
| Electrochemical Role in Batteries | Anode material in lead-acid and lithium batteries, enhances capacity and cycle stability | Cathode active material in NiMH and NiCd batteries; offers high energy density and corrosion resistance |
| Abundance and Cost | Moderately abundant; lower cost | Abundant; moderate cost |
| Corrosion Resistance | Moderate | High |
| Environmental Impact | Toxic in high quantities; requires careful handling | Less toxic; recyclable and widely used |
Introduction to Antimony and Nickel in Battery Technology
Antimony and nickel both play critical roles in battery technology due to their unique electrochemical properties. Antimony enhances battery performance through its high volumetric capacity and stability when used in anodes, particularly in lithium-ion and sodium-ion batteries, boosting energy density and cycle life. Nickel, widely utilized in cathodes and battery chemistries such as nickel-metal hydride (NiMH) and nickel-cobalt-aluminum (NCA), provides superior conductivity and structural strength, contributing to higher power output and durability.
Chemical Properties and Abundance
Antimony exhibits higher electrical conductivity and greater theoretical specific capacity compared to nickel, making it advantageous for battery anodes, especially in sodium-ion batteries. Chemically, antimony undergoes significant volume changes during alloying with sodium, necessitating robust structural designs, while nickel offers better structural stability but lower capacity. In terms of abundance, nickel is more widely distributed and accessible globally, whereas antimony is relatively scarce, impacting large-scale battery production feasibility.
Role in Battery Chemistry
Antimony enhances battery performance by serving as an alloying element in lithium-ion and sodium-ion batteries, improving cycling stability and capacity through its ability to alloy with lithium or sodium ions. Nickel plays a crucial role in battery cathodes, especially in lithium nickel manganese cobalt oxide (NMC) and lithium nickel cobalt aluminum oxide (NCA) chemistries, by increasing energy density and enhancing charge capacity. The distinct electrochemical properties of antimony and nickel influence anode and cathode behavior respectively, making them vital for optimizing battery efficiency and lifespan.
Energy Density Comparison
Antimony exhibits a higher theoretical energy density compared to nickel, making it a promising anode material for next-generation batteries. The volumetric capacity of antimony can reach up to 660 mAh/cm3, surpassing nickel's typical capacity of around 500 mAh/cm3 in battery applications. This increased energy density potential positions antimony as a key candidate for improving battery performance in electric vehicles and portable electronics.
Cost and Resource Availability
Antimony is often more cost-effective than nickel due to its lower market price and abundant reserves, making it a viable option for large-scale battery production. Nickel, while essential for high-energy-density batteries, faces supply constraints and price volatility driven by mining concentration in specific regions. The widespread availability of antimony supports stable pricing and resource security, whereas nickel's limited geographic distribution can lead to supply risks impacting battery manufacturing costs.
Environmental Impact and Sustainability
Antimony mining and processing pose significant environmental challenges due to toxic byproducts, heavy metal contamination, and limited recycling options, impacting soil and water quality. Nickel, while also associated with mining-related environmental degradation, benefits from more established recycling infrastructure and advances in sustainable extraction methods that reduce ecological damage. The sustainability of battery production increasingly favors nickel-based chemistries due to lower toxicity and better circular economy potential compared to antimony-enhanced batteries.
Performance and Longevity
Antimony enhances battery performance by providing higher energy density and improved charge retention compared to nickel, which typically offers better corrosion resistance and thermal stability. Batteries incorporating antimony anodes exhibit longer cycle life due to reduced volume expansion and structural integrity. Nickel-based batteries generally have faster charge-discharge rates but may suffer from capacity degradation over extended use, making antimony a favorable choice for applications demanding superior longevity and consistent performance.
Industrial Applications and Trends
Antimony enhances battery performance by improving lead-acid battery plates' durability and charge efficiency, making it valuable in industrial applications such as backup power systems and heavy machinery. Nickel contributes to the development of high-energy-density batteries, especially in nickel-metal hydride (NiMH) and nickel-cadmium (NiCd) batteries, widely used in electric vehicles and renewable energy storage. Current trends show increasing industrial demand for nickel-based batteries due to their longer life cycles and environmental benefits, while antimony remains critical for specialized applications requiring high corrosion resistance.
Safety and Thermal Stability
Antimony exhibits lower thermal stability than nickel, leading to increased risks of overheating and thermal runaway in battery applications. Nickel offers superior safety performance due to its higher melting point and better resistance to thermal degradation under high charge-discharge cycles. The enhanced thermal stability of nickel-based batteries reduces the likelihood of fire hazards and improves overall battery longevity compared to antimony-based systems.
Future Prospects and Research Directions
Antimony offers high theoretical capacity and excellent cycling stability, making it a promising anode material for next-generation sodium-ion and lithium-ion batteries. Nickel, known for its high energy density and conductivity, remains crucial in cathode development, particularly in layered oxide and nickel-rich cathodes for electric vehicles. Future research focuses on enhancing antimony's volumetric expansion management and exploring nickel's sustainable sourcing and recycling to improve battery performance and environmental impact.
Infographic: Antimony vs Nickel for Battery
 azmater.com
azmater.com