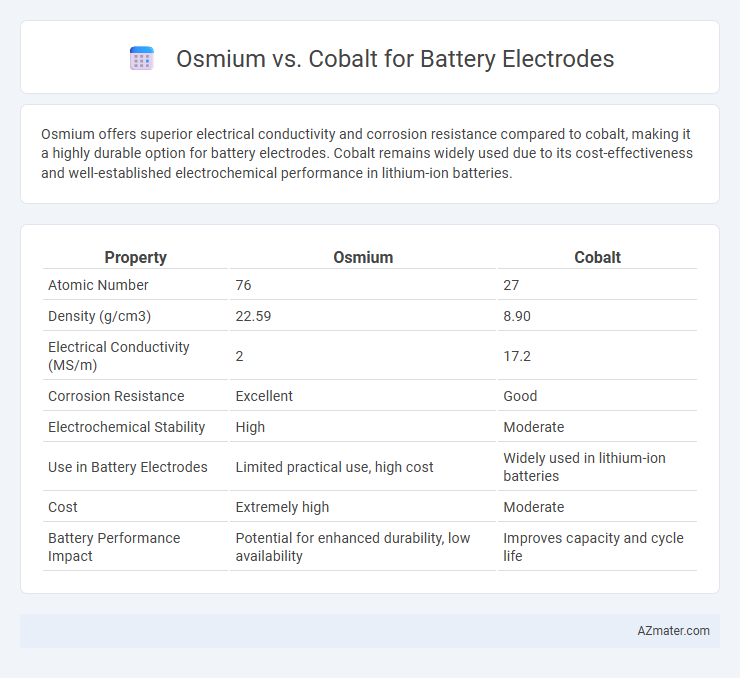
Osmium offers superior electrical conductivity and corrosion resistance compared to cobalt, making it a highly durable option for battery electrodes. Cobalt remains widely used due to its cost-effectiveness and well-established electrochemical performance in lithium-ion batteries.
Table of Comparison
| Property | Osmium | Cobalt |
|---|---|---|
| Atomic Number | 76 | 27 |
| Density (g/cm3) | 22.59 | 8.90 |
| Electrical Conductivity (MS/m) | 2 | 17.2 |
| Corrosion Resistance | Excellent | Good |
| Electrochemical Stability | High | Moderate |
| Use in Battery Electrodes | Limited practical use, high cost | Widely used in lithium-ion batteries |
| Cost | Extremely high | Moderate |
| Battery Performance Impact | Potential for enhanced durability, low availability | Improves capacity and cycle life |
Introduction to Osmium and Cobalt in Battery Electrodes
Osmium and cobalt are transition metals with distinct electrochemical properties influencing their application in battery electrodes. Osmium offers high density and excellent catalytic activity, potentially enhancing electron transfer rates and improving energy density in electrode materials. Cobalt, widely used in lithium-ion batteries, provides stable cycling performance and high conductivity, making it a critical component in cathode formulations like lithium cobalt oxide (LiCoO2).
Elemental Properties: Osmium vs Cobalt
Osmium and cobalt exhibit distinct elemental properties influencing their suitability as battery electrode materials. Osmium boasts a high density (22.59 g/cm3) and exceptional hardness, contributing to robust electrode structures but posing challenges for ion diffusion and cost-efficiency. In contrast, cobalt offers moderate density (8.90 g/cm3), good electrical conductivity, and well-established electrochemical activity, making it a preferred choice for stable, high-performance battery electrodes.
Electrochemical Performance Comparison
Osmium electrodes exhibit superior electrochemical stability and higher catalytic activity than cobalt in battery applications, resulting in enhanced charge-discharge efficiency and longer cycle life. The higher electron density and corrosion resistance of osmium improve electrode conductivity and reduce capacity fading under prolonged use. Conversely, cobalt offers cost-effectiveness and adequate performance, but its electrochemical properties, including lower overpotential and smaller voltage hysteresis, are generally outperformed by osmium in advanced battery systems.
Energy Density and Capacity Analysis
Osmium exhibits higher energy density due to its superior atomic weight and electron configuration, enabling greater charge storage compared to cobalt. Cobalt remains prevalent in lithium-ion battery electrodes for its balanced capacity and electrochemical stability, though osmium's potential for enhanced capacity warrants further research. Comparative studies indicate osmium-based electrodes could achieve increased energy density but face challenges in cost and material availability.
Cycle Life and Stability Factors
Osmium electrodes exhibit superior cycle life and electrochemical stability due to their high corrosion resistance and dense atomic structure, which minimizes degradation during repeated charge-discharge cycles. In contrast, cobalt electrodes, while offering good conductivity and capacity, tend to suffer from faster capacity fading and structural breakdown under prolonged cycling. The enhanced durability of osmium makes it a promising candidate for improving battery longevity, particularly in demanding applications requiring sustained stability.
Cost and Resource Availability
Osmium is significantly more expensive than cobalt due to its rarity and complex extraction processes, making it less feasible for widespread battery electrode applications. Cobalt, while costly, benefits from a more established supply chain and higher natural abundance, especially in countries like the Democratic Republic of Congo. The limited availability and elevated price of osmium restrict its scalability, whereas cobalt remains a more economically viable option for mass-market battery electrodes.
Environmental and Safety Considerations
Osmium and cobalt differ significantly in environmental impact and safety for battery electrodes, with cobalt raising major concerns due to its toxic mining byproducts and associated human health risks. Osmium, while less commonly used, presents challenges related to its rarity and the potential release of toxic osmium tetroxide gas during processing. Sustainable battery development prioritizes minimizing cobalt dependency due to its ethical and environmental hazards, while ensuring safe handling protocols for osmium to mitigate toxic exposure.
Manufacturing Challenges and Scalability
Osmium's high density and rarity pose significant manufacturing challenges and scalability issues for battery electrode production, making it costly and limited in large-scale applications. Cobalt, while more abundant, faces ethical sourcing problems and price volatility, impacting consistent manufacturing and scalability. Advances in cobalt recycling and alternative sourcing are crucial for improving its sustainability in electrode manufacturing processes.
Current Research and Market Trends
Osmium exhibits remarkable catalytic efficiency and stability in battery electrode applications, making it an attractive yet expensive material compared to cobalt, which remains dominant due to its cost-effectiveness and established supply chains. Current research emphasizes enhancing osmium's electrode performance through nanostructuring techniques to improve energy density and cycle life while addressing toxicity concerns. Market trends indicate a gradual shift towards cobalt alternatives, including osmium-based composites, driven by sustainability demands and cobalt supply volatility from geopolitical factors.
Future Prospects for Osmium and Cobalt Electrodes
Osmium exhibits exceptional catalytic properties and high electrical conductivity, making it a promising candidate for next-generation battery electrodes, particularly in enhancing energy density and charge-discharge efficiency. Cobalt remains a critical component in lithium-ion batteries due to its stability and excellent electrochemical performance, but concerns over supply constraints and ethical sourcing drive research into alternatives like osmium. Advancements in nanotechnology and electrode design could enable osmium to overcome its current cost limitations, fostering its adoption alongside or as a substitute for cobalt in future high-performance batteries.
Infographic: Osmium vs Cobalt for Battery Electrode
 azmater.com
azmater.com