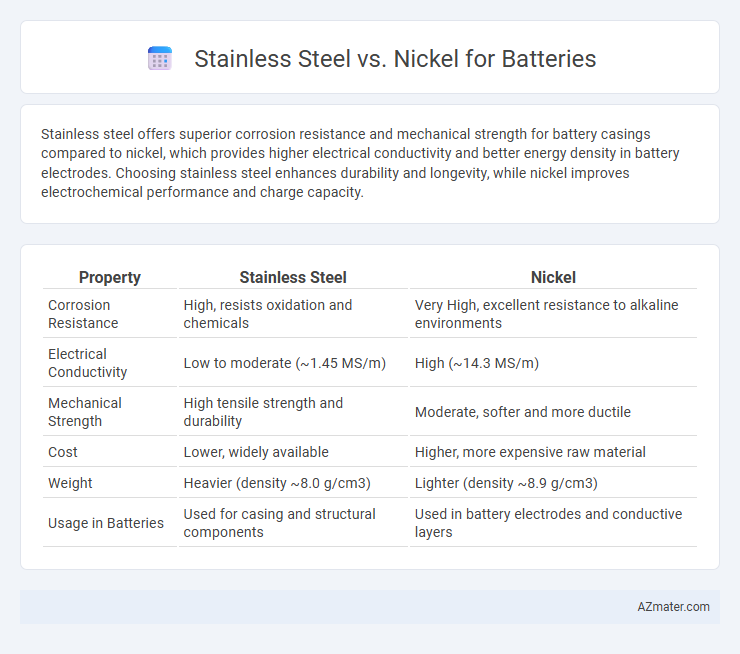
Stainless steel offers superior corrosion resistance and mechanical strength for battery casings compared to nickel, which provides higher electrical conductivity and better energy density in battery electrodes. Choosing stainless steel enhances durability and longevity, while nickel improves electrochemical performance and charge capacity.
Table of Comparison
| Property | Stainless Steel | Nickel |
|---|---|---|
| Corrosion Resistance | High, resists oxidation and chemicals | Very High, excellent resistance to alkaline environments |
| Electrical Conductivity | Low to moderate (~1.45 MS/m) | High (~14.3 MS/m) |
| Mechanical Strength | High tensile strength and durability | Moderate, softer and more ductile |
| Cost | Lower, widely available | Higher, more expensive raw material |
| Weight | Heavier (density ~8.0 g/cm3) | Lighter (density ~8.9 g/cm3) |
| Usage in Batteries | Used for casing and structural components | Used in battery electrodes and conductive layers |
Introduction: Stainless Steel vs Nickel in Battery Technology
Stainless steel and nickel serve critical roles in battery technology, with stainless steel valued for its corrosion resistance and mechanical strength, while nickel is prized for its excellent electrochemical properties and conductivity. Nickel is widely used in battery cathodes, particularly in nickel-metal hydride (NiMH) and lithium-ion batteries, enhancing energy density and cycle life. Stainless steel commonly functions as a durable casing material, providing structural integrity and protecting internal components from environmental damage.
Material Composition and Properties
Stainless steel features an iron-based alloy composition with chromium (typically 10-30%) that forms a passive oxide layer, enhancing corrosion resistance and mechanical strength critical for durable battery casings. Nickel, a pure metal or alloyed element in battery components, offers superior electrical conductivity and excellent resistance to oxidation, making it ideal for electrode materials in nickel-metal hydride and lithium-ion batteries. The distinct material properties influence battery performance, where stainless steel contributes structural stability while nickel optimizes electrochemical efficiency.
Role in Battery Performance
Stainless steel enhances battery performance by providing excellent corrosion resistance and mechanical strength, which ensures durability and safety in battery casings and connectors. Nickel plays a crucial role in battery electrodes, particularly in nickel-metal hydride (NiMH) and lithium-ion batteries, by facilitating efficient electron transfer and boosting energy density. The choice between stainless steel and nickel impacts overall battery efficiency, longevity, and thermal stability crucial for high-performance energy storage solutions.
Corrosion Resistance and Longevity
Stainless steel exhibits superior corrosion resistance compared to nickel, making it ideal for battery components exposed to harsh environments and electrolytes. Nickel offers high electrical conductivity but tends to corrode faster under acidic or alkaline battery conditions, potentially reducing the battery's lifespan. Stainless steel's durability and resistance to oxidation enhance battery longevity by maintaining structural integrity over extended charge-discharge cycles.
Electrical Conductivity Comparison
Stainless steel typically exhibits electrical conductivity around 1.45 million siemens per meter, significantly lower than nickel's conductivity of approximately 14 million siemens per meter. This makes nickel a preferred material for battery electrodes and connectors due to its superior ability to efficiently conduct current. In battery applications, nickel's high conductivity enhances charge transfer and overall performance, whereas stainless steel's limited conductivity may result in higher internal resistance and reduced efficiency.
Cost and Availability
Stainless steel offers a cost-effective and widely available solution for battery components, with robust corrosion resistance and mechanical strength suitable for diverse applications. Nickel, while more expensive and less abundant, provides superior conductivity and higher energy density in batteries, often justifying the premium in high-performance devices. The cost difference between stainless steel and nickel significantly impacts large-scale battery manufacturing, where material availability influences production scalability.
Manufacturing and Processing Considerations
Stainless steel offers superior corrosion resistance and mechanical strength, making it ideal for battery casings that require durability and long-term stability during manufacturing. Nickel excels in electrical conductivity and ease of plating, which enhances battery electrode performance but demands precise control over deposition processes to avoid contamination. Both materials require tailored processing methods; stainless steel often undergoes cold rolling and passivation, while nickel benefits from electroplating techniques that optimize surface properties for battery efficiency.
Environmental Impact and Recyclability
Stainless steel offers superior recyclability with a well-established recycling infrastructure that significantly reduces environmental impact through material recovery and reduced resource extraction. Nickel, while essential for battery performance, has a higher environmental footprint due to energy-intensive mining processes and limited recycling efficiency. Sustainable battery design increasingly favors stainless steel to minimize ecological damage and promote circular economy practices in battery manufacturing.
Industry Applications and Use Cases
Stainless steel offers superior corrosion resistance and mechanical strength, making it ideal for battery housings and structural components in automotive and industrial energy storage systems. Nickel's high electrochemical stability and excellent conductivity are crucial for battery electrodes, especially in nickel-metal hydride (NiMH) and lithium-ion battery cathodes used in consumer electronics and electric vehicles. In industry applications, stainless steel is preferred for durable battery enclosures, while nickel is essential for optimizing energy density and cycle life in battery chemistries.
Conclusion: Choosing the Right Material
Selecting stainless steel for battery casings offers superior corrosion resistance and mechanical strength, making it ideal for long-lasting, durable applications. Nickel provides excellent electrical conductivity and enhances battery performance but may be prone to higher costs and potential corrosion in certain environments. Evaluating the specific requirements of conductivity, durability, and cost-efficiency is essential to determine the optimal material for battery construction.
Infographic: Stainless steel vs Nickel for Battery
 azmater.com
azmater.com