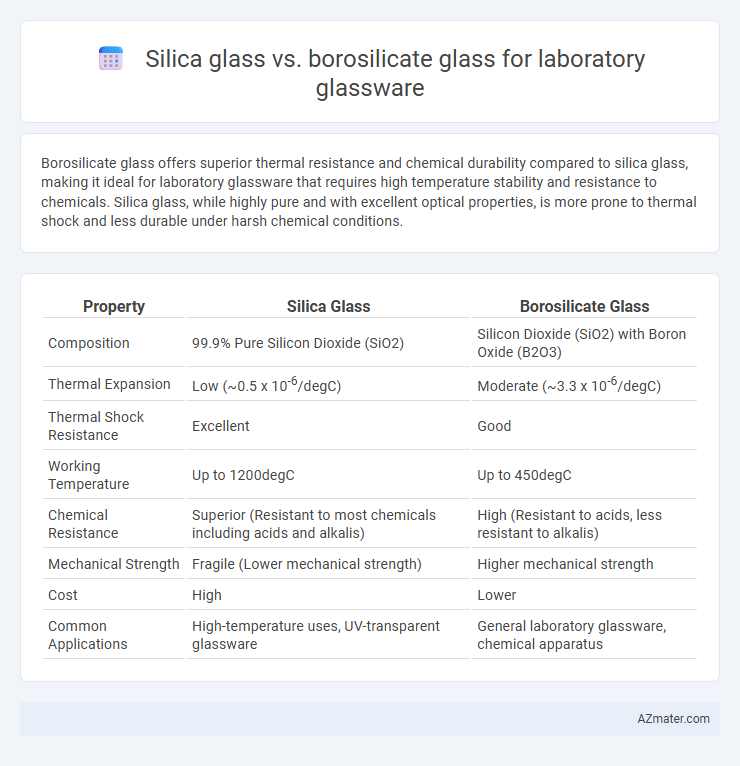
Borosilicate glass offers superior thermal resistance and chemical durability compared to silica glass, making it ideal for laboratory glassware that requires high temperature stability and resistance to chemicals. Silica glass, while highly pure and with excellent optical properties, is more prone to thermal shock and less durable under harsh chemical conditions.
Table of Comparison
| Property | Silica Glass | Borosilicate Glass |
|---|---|---|
| Composition | 99.9% Pure Silicon Dioxide (SiO2) | Silicon Dioxide (SiO2) with Boron Oxide (B2O3) |
| Thermal Expansion | Low (~0.5 x 10-6/degC) | Moderate (~3.3 x 10-6/degC) |
| Thermal Shock Resistance | Excellent | Good |
| Working Temperature | Up to 1200degC | Up to 450degC |
| Chemical Resistance | Superior (Resistant to most chemicals including acids and alkalis) | High (Resistant to acids, less resistant to alkalis) |
| Mechanical Strength | Fragile (Lower mechanical strength) | Higher mechanical strength |
| Cost | High | Lower |
| Common Applications | High-temperature uses, UV-transparent glassware | General laboratory glassware, chemical apparatus |
Introduction to Laboratory Glassware Materials
Silica glass offers exceptional thermal resistance and chemical durability, making it ideal for high-temperature laboratory applications where purity and minimal contamination are critical. Borosilicate glass features excellent thermal shock resistance and chemical stability, providing durability for routine laboratory procedures involving rapid temperature changes. Both materials are essential in laboratory glassware, with silica glass favored for precision tasks and borosilicate glass for general-purpose use due to its superior mechanical strength and affordability.
Overview of Silica Glass Properties
Silica glass, composed primarily of pure silicon dioxide (SiO2), exhibits exceptional thermal stability with a high melting point around 1713degC, making it ideal for high-temperature laboratory applications. Its low thermal expansion coefficient (approximately 0.5 x 10^-6 /degC) minimizes stress and fracture under rapid temperature changes, surpassing the thermal shock resistance of borosilicate glass. Chemically inert and highly transparent to ultraviolet light, silica glass is preferred for experiments requiring optical clarity and durability against aggressive chemicals.
Overview of Borosilicate Glass Properties
Borosilicate glass, known for its excellent thermal resistance and chemical durability, typically contains about 12-13% boron oxide, which imparts superior shock resistance compared to silica glass. Its low coefficient of thermal expansion (approximately 3.3 x 10-6 /degC) minimizes thermal stress, making it ideal for laboratory glassware subjected to rapid temperature changes. High resistance to alkalis and acids ensures borosilicate glass maintains structural integrity and clarity during demanding chemical experiments.
Thermal Resistance Comparison
Silica glass exhibits superior thermal resistance, withstanding temperatures up to 1700degC, making it ideal for high-temperature laboratory applications. Borosilicate glass, capable of tolerating temperatures around 450degC to 500degC, offers excellent thermal shock resistance but lower maximum temperature tolerance than silica glass. The choice between silica and borosilicate glass depends on the specific thermal requirements of laboratory experiments and equipment.
Chemical Durability: Silica vs Borosilicate
Silica glass exhibits superior chemical durability against strong acids and bases, maintaining stability in harsh laboratory environments compared to borosilicate glass. Borosilicate glass offers good resistance to thermal shock and moderate chemical corrosion but is more susceptible to alkalis and hydrofluoric acid. Silica glass's higher purity and chemical inertness make it the preferred choice for applications requiring exceptional resistance to aggressive chemicals.
Transparency and Optical Clarity Differences
Silica glass exhibits superior transparency and optical clarity compared to borosilicate glass, making it ideal for applications requiring high light transmission and minimal distortion. Its high purity and low iron content result in increased UV transparency, essential for precise spectroscopic analysis. Borosilicate glass, while chemically durable and resistant to thermal shock, has lower optical clarity with slight coloration and reduced UV transparency due to its boron oxide content.
Mechanical Strength and Durability
Silica glass offers superior mechanical strength with high resistance to thermal shock and chemical corrosion, making it ideal for high-temperature laboratory applications. Borosilicate glass provides excellent durability and enhanced resistance to mechanical impact, with a lower coefficient of thermal expansion that reduces the risk of cracking under rapid temperature changes. Both materials ensure reliable performance, but silica glass excels in extreme conditions while borosilicate glass delivers balanced strength and durability for general lab use.
Cost Analysis and Availability
Silica glass offers superior thermal resistance and chemical durability but comes at a higher cost, making it less commonly used for standard laboratory glassware compared to borosilicate glass. Borosilicate glass is more affordable and widely available, with excellent thermal shock resistance and chemical stability suitable for most lab applications. The cost-effectiveness and easy accessibility of borosilicate glass make it the preferred choice for routine laboratory use.
Application Suitability in Laboratory Settings
Silica glass offers superior thermal resistance and chemical inertness, making it highly suitable for high-temperature applications and reactions involving aggressive chemicals in laboratory settings. Borosilicate glass provides excellent thermal shock resistance and durability, ideal for general-purpose labware such as beakers, flasks, and condensers exposed to rapid temperature changes. Selection depends on experimental conditions, with silica glass preferred for extreme environments and borosilicate for versatile, everyday laboratory use.
Conclusion: Choosing the Right Glass for Your Needs
Silica glass offers superior thermal resistance and chemical inertness, making it ideal for high-temperature applications and aggressive chemical reactions in laboratory settings. Borosilicate glass provides excellent thermal shock resistance and is more cost-effective, suitable for general-purpose labware requiring moderate heat endurance and durability. Selecting between silica and borosilicate glass hinges on the specific experimental conditions, with silica glass preferred for extreme environments and borosilicate for everyday laboratory use.
Infographic: Silica glass vs Borosilicate glass for Laboratory glassware
 azmater.com
azmater.com