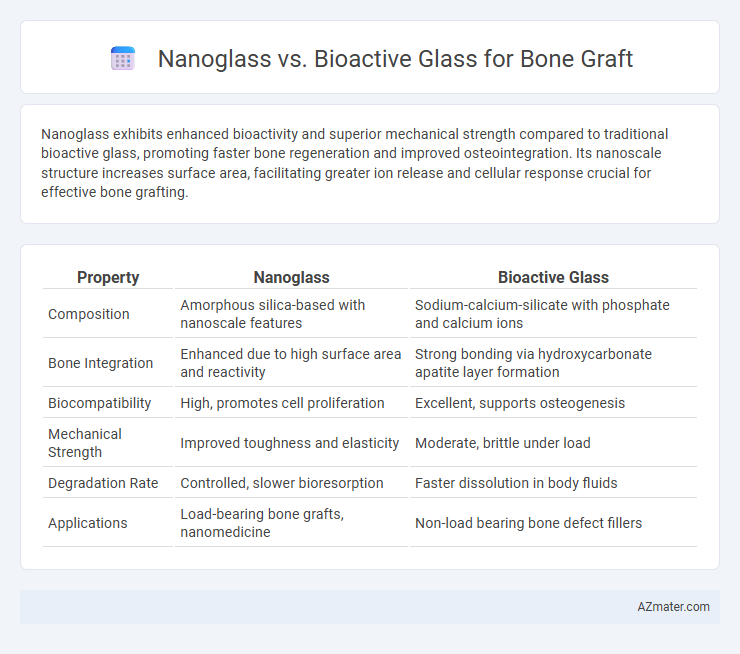
Nanoglass exhibits enhanced bioactivity and superior mechanical strength compared to traditional bioactive glass, promoting faster bone regeneration and improved osteointegration. Its nanoscale structure increases surface area, facilitating greater ion release and cellular response crucial for effective bone grafting.
Table of Comparison
| Property | Nanoglass | Bioactive Glass |
|---|---|---|
| Composition | Amorphous silica-based with nanoscale features | Sodium-calcium-silicate with phosphate and calcium ions |
| Bone Integration | Enhanced due to high surface area and reactivity | Strong bonding via hydroxycarbonate apatite layer formation |
| Biocompatibility | High, promotes cell proliferation | Excellent, supports osteogenesis |
| Mechanical Strength | Improved toughness and elasticity | Moderate, brittle under load |
| Degradation Rate | Controlled, slower bioresorption | Faster dissolution in body fluids |
| Applications | Load-bearing bone grafts, nanomedicine | Non-load bearing bone defect fillers |
Introduction to Bone Graft Materials
Nanoglass and bioactive glass are advanced materials used for bone grafts, offering enhanced osteoconductivity and bioactivity critical for bone regeneration. Nanoglass features nanoscale particles that improve cell adhesion and proliferation, while bioactive glass interacts chemically with bone tissue to stimulate new bone formation. Both materials serve as effective scaffolds in orthopedic and dental applications, promoting faster healing and integration.
Overview of Nanoglass
Nanoglass is an advanced form of bioactive glass characterized by its nanoscale particle size, which significantly enhances its surface area and reactivity compared to conventional bioactive glass. This increased surface area promotes faster hydroxyapatite layer formation, improving osteointegration and bone regeneration in grafting applications. Nanoglass also exhibits superior mechanical strength and bioactivity, making it a promising material for bone grafts with accelerated healing and improved biocompatibility.
Understanding Bioactive Glass
Bioactive glass is a silica-based material known for its exceptional bone bonding capabilities and ability to stimulate osteogenesis through the release of biologically active ions like calcium and phosphate. Compared to nanoglass, bioactive glass offers a well-established track record in clinical bone grafting applications due to its high bioactivity and superior integration with native bone tissue. Its controlled degradation and ion release promote faster bone regeneration and enhanced mechanical stability in graft sites.
Composition and Structure Comparison
Nanoglass and bioactive glass differ primarily in their compositional nano-scale features and structural attributes impacting bone graft performance. Nanoglass consists of ultra-fine particles typically less than 100 nm, enhancing its surface area and reactivity, while bioactive glass is composed of silica-based matrices with specific ratios of SiO2, CaO, and P2O5 promoting osteoconductivity. The amorphous structure of nanoglass allows faster ion release and improved bioactivity, whereas traditional bioactive glass features a controlled crystallinity that supports gradual bone bonding and integration.
Mechanisms of Bone Regeneration
Nanoglass promotes bone regeneration through its nanoscale surface topography, enhancing osteoblast adhesion and proliferation, while releasing bioactive ions that stimulate cellular signaling pathways for new bone matrix formation. Bioactive glass facilitates bone repair by forming a hydroxycarbonate apatite layer upon implantation, which bonds directly with bone and activates osteogenic cells through ionic dissolution products. Both materials leverage ion release to modulate gene expression critical for osteogenesis, but nanoglass's nanoscale features provide superior cellular interaction and faster mineralization rates.
Bioactivity and Osteointegration Differences
Nanoglass exhibits smaller particle size and higher surface area, enhancing ion release and stimulating faster bioactivity, which significantly promotes early-stage osteointegration in bone grafts. Bioactive glass, with its well-established hydroxyapatite layer formation, supports long-term bone bonding and gradual osteointegration through sustained ion exchange and cellular response. Compared to bioactive glass, nanoglass's enhanced reactivity results in more rapid bone cell proliferation and bio-mineralization, optimizing early healing phases in bone regeneration.
Mechanical Strength and Durability
Nanoglass exhibits superior mechanical strength compared to bioactive glass due to its nanoscale particle size and enhanced surface area, which promotes stronger bonding and load-bearing capacity in bone graft applications. Bioactive glass offers excellent biocompatibility and osteoconductivity but typically falls short in long-term durability under mechanical stress. The enhanced fracture toughness and resistance to degradation of nanoglass make it a more durable option for bone grafts requiring sustained structural support.
Clinical Applications and Case Studies
Nanoglass materials exhibit enhanced bioactivity and faster bone regeneration rates compared to bioactive glass, making them highly effective in clinical applications such as maxillofacial reconstruction and spinal fusion. Case studies demonstrate nanoglass's superior integration with host bone tissue, reduced healing time, and improved mechanical stability in load-bearing grafts. Bioactive glass remains widely used for its osteoconductive and antimicrobial properties, particularly in dental implants and cranial defect repairs, but nanoglass's nanoscale structure provides more favorable cellular responses in complex bone grafting scenarios.
Advantages and Limitations
Nanoglass offers superior bioactivity and enhanced osteoconductivity compared to traditional bioactive glass, promoting faster bone regeneration and improved cellular adhesion due to its nanoscale particle size. However, nanoglass faces limitations such as potential agglomeration, higher production costs, and challenges in maintaining consistent mechanical strength. Bioactive glass remains cost-effective with proven long-term clinical outcomes but exhibits slower degradation rates and less surface area for cell interaction than nanoglass.
Future Trends in Bone Grafting Technology
Nanoglass and bioactive glass represent cutting-edge materials in bone grafting, each offering distinct regenerative advantages through enhanced osteoconductivity and biocompatibility. Future trends in bone grafting technology emphasize the integration of nanoglass with bioactive glass composites to synergistically improve bone healing rates and mechanical strength while minimizing immune responses. Advances in 3D printing and biofabrication are expected to enable customizable, patient-specific grafts combining nanoglass's nanoscale properties with bioactive glass's ionic release for optimized bone regeneration.
Infographic: Nanoglass vs Bioactive glass for Bone graft
 azmater.com
azmater.com