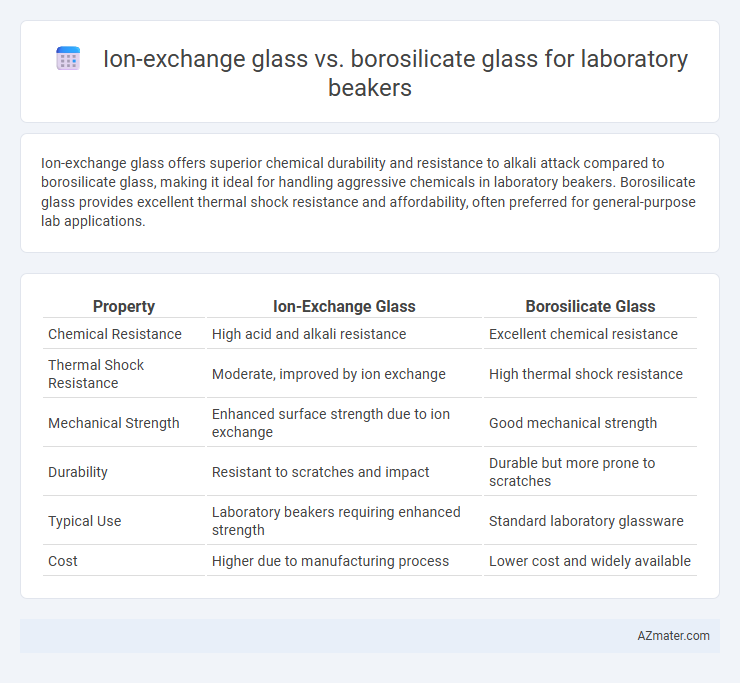
Ion-exchange glass offers superior chemical durability and resistance to alkali attack compared to borosilicate glass, making it ideal for handling aggressive chemicals in laboratory beakers. Borosilicate glass provides excellent thermal shock resistance and affordability, often preferred for general-purpose lab applications.
Table of Comparison
| Property | Ion-Exchange Glass | Borosilicate Glass |
|---|---|---|
| Chemical Resistance | High acid and alkali resistance | Excellent chemical resistance |
| Thermal Shock Resistance | Moderate, improved by ion exchange | High thermal shock resistance |
| Mechanical Strength | Enhanced surface strength due to ion exchange | Good mechanical strength |
| Durability | Resistant to scratches and impact | Durable but more prone to scratches |
| Typical Use | Laboratory beakers requiring enhanced strength | Standard laboratory glassware |
| Cost | Higher due to manufacturing process | Lower cost and widely available |
Introduction to Laboratory Glassware Materials
Ion-exchange glass offers enhanced chemical durability and thermal resistance compared to standard borosilicate glass, making it ideal for laboratory beakers that require high resistance to alkalis and acids. Borosilicate glass is favored for its excellent thermal shock resistance and clarity, widely used for general laboratory applications due to its affordability and reliable performance. Selection between ion-exchange and borosilicate glass depends on the specific experimental conditions, such as exposure to corrosive chemicals or thermal cycling requirements.
Overview of Ion-Exchange Glass
Ion-exchange glass is chemically strengthened through a process that replaces smaller ions in the glass surface with larger potassium ions, significantly improving its mechanical strength and resistance to thermal shock compared to traditional borosilicate glass. This enhanced durability makes ion-exchange glass ideal for laboratory beakers subjected to rapid temperature changes and mechanical stress. While borosilicate glass is known for its excellent chemical resistance and thermal stability, ion-exchange glass offers superior impact resistance, extending the lifespan and safety of laboratory glassware.
Borosilicate Glass: Properties and Applications
Borosilicate glass is highly valued for laboratory beakers due to its exceptional thermal resistance, low thermal expansion (approximately 3.3 x 10-6 /degC), and excellent chemical durability against acids and alkalis. Its superior heat tolerance allows repeated heating and cooling without cracking, making it ideal for precise experiments and high-temperature processes. Commonly used in chemistry, biology, and pharmaceutical labs, borosilicate glass beakers ensure reliable performance in applications requiring thermal shock resistance and chemical inertness.
Chemical Resistance: Ion-Exchange vs Borosilicate Glass
Ion-exchange glass offers superior chemical resistance due to its altered surface layer that enhances durability against acids and alkalis, making it less prone to leaching and surface corrosion. Borosilicate glass is highly resistant to thermal shock and general chemical attack but can be more vulnerable to alkali attack over prolonged exposure. For laboratory beakers, ion-exchange glass provides enhanced longevity and reliability in harsh chemical environments compared to standard borosilicate glass.
Thermal Shock Resistance of Laboratory Beakers
Ion-exchange glass laboratory beakers offer superior thermal shock resistance compared to borosilicate glass, due to the compressive stress layer created by the ion-exchange process, which significantly enhances durability under rapid temperature changes. Borosilicate glass, while known for its chemical resistance and moderate thermal stability, is more prone to cracking when exposed to sudden temperature shifts beyond its threshold of approximately 165degC. The enhanced thermal shock resistance of ion-exchange glass makes it ideal for applications involving frequent heating and cooling cycles, reducing the risk of breakage and improving safety in laboratory environments.
Mechanical Strength and Durability Comparison
Ion-exchange glass offers significantly higher mechanical strength and enhanced resistance to thermal and mechanical shocks compared to borosilicate glass, making it suitable for demanding laboratory environments. Borosilicate glass is valued for its chemical resistance and thermal stability but exhibits lower fracture toughness and is more prone to breakage under impact stress. The superior durability of ion-exchange glass results from its surface compression layer, which improves scratch resistance and extends the lifespan of laboratory beakers under rigorous use.
Cost Efficiency and Availability
Ion-exchange glass offers enhanced chemical durability and scratch resistance compared to borosilicate glass, but it generally comes at a higher cost, impacting cost efficiency for laboratory beakers. Borosilicate glass is widely available, more affordable, and sufficiently resistant to thermal shock and chemical corrosion, making it the preferred choice for standard laboratory applications. Considering cost efficiency and availability, borosilicate glass remains the most practical option for general laboratory beakers.
Safety Considerations in Laboratory Settings
Ion-exchange glass offers enhanced chemical durability and resistance to thermal shock, reducing the risk of breakage and chemical contamination in laboratory beakers. Borosilicate glass is widely favored for its excellent thermal resistance and mechanical strength, providing reliable performance under varying temperature conditions common in laboratories. Safety considerations emphasize the lower brittleness and higher impact resistance of ion-exchange glass, while borosilicate glass remains the standard for withstanding rapid temperature changes.
Suitability for Specific Laboratory Applications
Ion-exchange glass offers superior chemical resistance and enhanced durability, making it ideal for handling aggressive chemicals and high-stress laboratory environments. Borosilicate glass provides excellent thermal shock resistance and is suitable for applications involving rapid temperature changes or heating processes. Selecting between these materials depends on specific lab requirements such as chemical compatibility, mechanical strength, and thermal stability.
Conclusion: Choosing the Right Glass for Your Laboratory Beakers
Ion-exchange glass offers superior chemical durability and scratch resistance, making it ideal for high-precision laboratory applications requiring long-term reliability. Borosilicate glass provides excellent thermal resistance and cost-effectiveness, suitable for general-purpose lab beakers handling thermal cycling or moderate chemical exposure. Selecting the right glass depends on the specific laboratory demands, with ion-exchange glass preferred for enhanced durability and borosilicate glass favored for its thermal stability and affordability.
Infographic: Ion-exchange glass vs Borosilicate glass for Laboratory beaker
 azmater.com
azmater.com