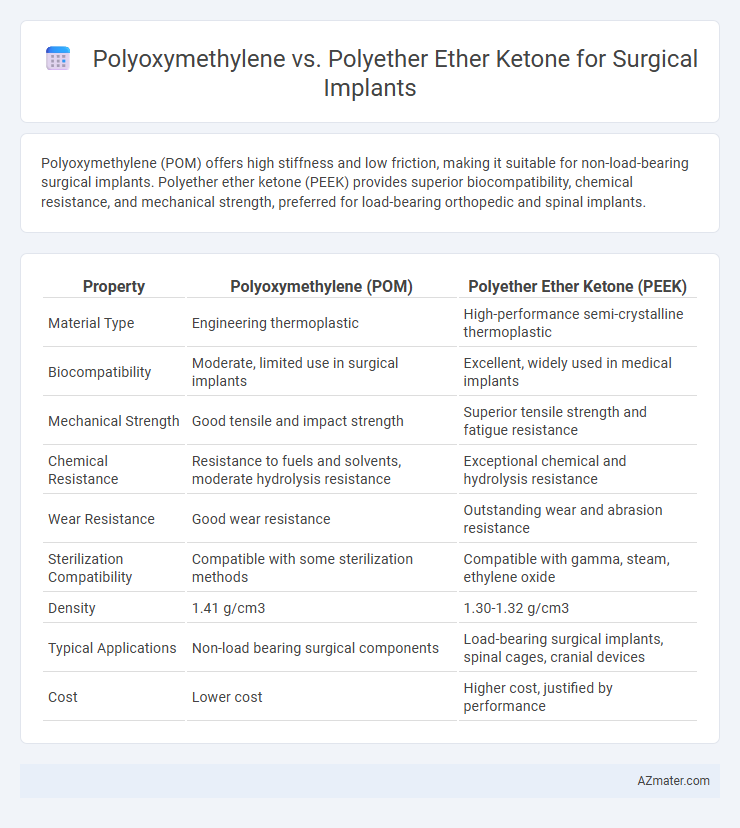
Polyoxymethylene (POM) offers high stiffness and low friction, making it suitable for non-load-bearing surgical implants. Polyether ether ketone (PEEK) provides superior biocompatibility, chemical resistance, and mechanical strength, preferred for load-bearing orthopedic and spinal implants.
Table of Comparison
| Property | Polyoxymethylene (POM) | Polyether Ether Ketone (PEEK) |
|---|---|---|
| Material Type | Engineering thermoplastic | High-performance semi-crystalline thermoplastic |
| Biocompatibility | Moderate, limited use in surgical implants | Excellent, widely used in medical implants |
| Mechanical Strength | Good tensile and impact strength | Superior tensile strength and fatigue resistance |
| Chemical Resistance | Resistance to fuels and solvents, moderate hydrolysis resistance | Exceptional chemical and hydrolysis resistance |
| Wear Resistance | Good wear resistance | Outstanding wear and abrasion resistance |
| Sterilization Compatibility | Compatible with some sterilization methods | Compatible with gamma, steam, ethylene oxide |
| Density | 1.41 g/cm3 | 1.30-1.32 g/cm3 |
| Typical Applications | Non-load bearing surgical components | Load-bearing surgical implants, spinal cages, cranial devices |
| Cost | Lower cost | Higher cost, justified by performance |
Introduction to Surgical Implant Materials
Polyoxymethylene (POM) and Polyether ether ketone (PEEK) are advanced polymers used in surgical implants, valued for their mechanical strength, biocompatibility, and resistance to wear. PEEK exhibits superior chemical stability and radiolucency, making it ideal for load-bearing orthopedic applications, while POM provides excellent stiffness and low friction in non-load-bearing implants. Both materials contribute significantly to the development of durable, high-performance surgical devices tailored to specific medical requirements.
Overview of Polyoxymethylene (POM)
Polyoxymethylene (POM), also known as acetal, is a highly crystalline thermoplastic characterized by excellent mechanical strength, stiffness, and low friction, making it suitable for surgical implants requiring durability and precision. Its biocompatibility and resistance to bodily fluids enhance its performance in medical applications, while maintaining stability under sterilization processes such as autoclaving. Compared to Polyether ether ketone (PEEK), POM offers a more cost-effective option, although PEEK demonstrates superior chemical resistance and higher thermal stability for long-term implant use.
Overview of Polyether Ether Ketone (PEEK)
Polyether Ether Ketone (PEEK) is a high-performance thermoplastic widely utilized in surgical implants due to its exceptional biocompatibility, chemical resistance, and mechanical strength. Compared to Polyoxymethylene (POM), PEEK offers superior fatigue resistance, radiolucency, and a modulus of elasticity closer to that of cortical bone, minimizing stress shielding in orthopedic applications. Its stability under sterilization processes and ability to support osseointegration make PEEK a preferred material for load-bearing surgical implants such as spinal cages and joint replacements.
Mechanical Properties: POM vs PEEK
Polyoxymethylene (POM) exhibits high stiffness and excellent dimensional stability, making it suitable for applications requiring rigidity and precision in surgical implants. Polyether ether ketone (PEEK) surpasses POM in tensile strength, fatigue resistance, and biocompatibility, offering superior mechanical performance under cyclic loading and long-term implantation conditions. The superior chemical resistance and higher melting point of PEEK contribute to its enhanced durability and reliability in demanding surgical environments compared to POM.
Biocompatibility and Bio-inertness Comparison
Polyoxymethylene (POM) demonstrates excellent mechanical strength and good biocompatibility but has moderate bio-inertness due to its potential for slight inflammatory response in vivo. Polyether ether ketone (PEEK) exhibits superior biocompatibility and bio-inertness, showing minimal tissue reaction and favorable osseointegration in surgical implant applications. PEEK's chemical stability and resistance to hydrolysis make it more suitable than POM for long-term implantation where sustained bio-inertness is critical.
Chemical Resistance in Surgical Environments
Polyoxymethylene (POM) exhibits moderate chemical resistance but can degrade when exposed to strong acids and sterilization chemicals common in surgical environments. Polyether ether ketone (PEEK) offers superior chemical resistance, maintaining structural integrity against aggressive sterilants like gamma radiation, ethylene oxide, and autoclave steam. PEEK's robustness in chemical stability makes it the preferred material for long-term surgical implants requiring high durability and biocompatibility.
Wear and Fatigue Performance
Polyoxymethylene (POM) exhibits excellent wear resistance and low friction, making it suitable for surgical implants subjected to repetitive motion, but its fatigue performance can be limited under prolonged cyclic loading. Polyether ether ketone (PEEK) offers superior fatigue strength and outstanding wear resistance, maintaining structural integrity even under high-stress and long-term orthopedic applications. PEEK's biocompatibility and mechanical stability under repetitive loading cycles generally outperform POM, ensuring enhanced durability in surgical implants.
Sterilization Compatibility
Polyoxymethylene (POM) exhibits good resistance to steam sterilization methods like autoclaving but may degrade under prolonged exposure to high temperatures and aggressive sterilants. Polyether ether ketone (PEEK) offers superior thermal stability and chemical resistance, maintaining structural integrity after repeated sterilization cycles including autoclaving, gamma irradiation, and ethylene oxide exposure. PEEK's biocompatibility and sterilization compatibility make it a preferred material for long-term surgical implants requiring rigorous sterilization protocols.
Cost Effectiveness and Accessibility
Polyoxymethylene (POM) offers superior cost effectiveness and greater accessibility compared to Polyether ether ketone (PEEK) for surgical implants due to its lower raw material and manufacturing costs, making it suitable for a wider range of healthcare facilities. PEEK exhibits exceptional biocompatibility, chemical resistance, and mechanical strength but presents a significantly higher price point and limited availability in some markets, restricting its use primarily to high-end or specialized surgical applications. The choice between POM and PEEK hinges on balancing budget constraints with performance requirements, with POM favored in cost-sensitive settings and PEEK reserved for implants demanding advanced durability and biological compatibility.
Application Trends and Recommendations for Surgeons
Polyether ether ketone (PEEK) is increasingly preferred over polyoxymethylene (POM) for surgical implants due to its superior biocompatibility, chemical resistance, and mechanical strength, particularly in spinal and orthopedic applications. Trends indicate growing use of PEEK in load-bearing implants where long-term stability and imaging compatibility, such as MRI transparency, are critical. Surgeons are recommended to select PEEK for implants requiring high durability and minimal inflammatory response, reserving POM for non-load-bearing devices or temporary applications where cost-effectiveness is a priority.
Infographic: Polyoxymethylene vs Polyether ether ketone for Surgical implant
 azmater.com
azmater.com