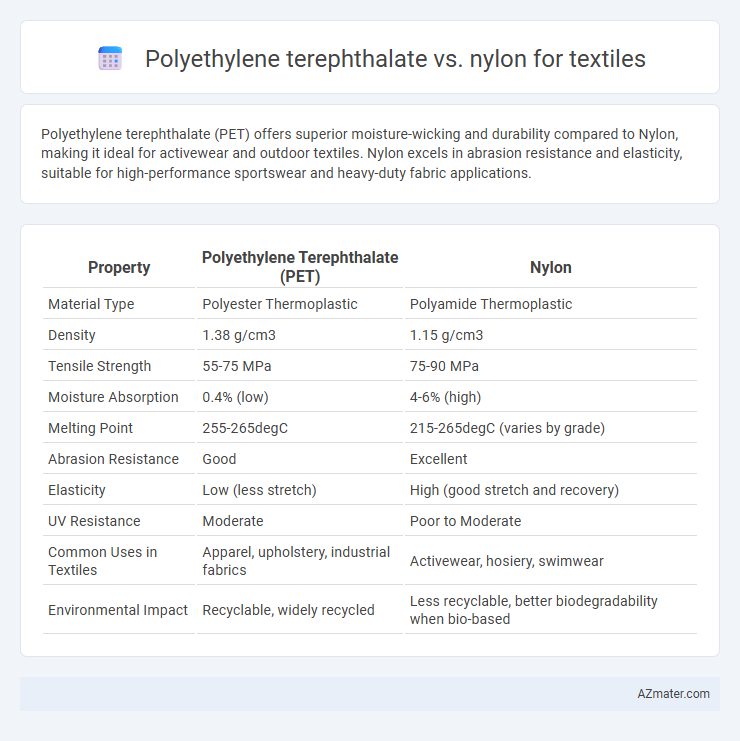
Polyethylene terephthalate (PET) offers superior moisture-wicking and durability compared to Nylon, making it ideal for activewear and outdoor textiles. Nylon excels in abrasion resistance and elasticity, suitable for high-performance sportswear and heavy-duty fabric applications.
Table of Comparison
| Property | Polyethylene Terephthalate (PET) | Nylon |
|---|---|---|
| Material Type | Polyester Thermoplastic | Polyamide Thermoplastic |
| Density | 1.38 g/cm3 | 1.15 g/cm3 |
| Tensile Strength | 55-75 MPa | 75-90 MPa |
| Moisture Absorption | 0.4% (low) | 4-6% (high) |
| Melting Point | 255-265degC | 215-265degC (varies by grade) |
| Abrasion Resistance | Good | Excellent |
| Elasticity | Low (less stretch) | High (good stretch and recovery) |
| UV Resistance | Moderate | Poor to Moderate |
| Common Uses in Textiles | Apparel, upholstery, industrial fabrics | Activewear, hosiery, swimwear |
| Environmental Impact | Recyclable, widely recycled | Less recyclable, better biodegradability when bio-based |
Introduction to Polyethylene Terephthalate (PET) and Nylon
Polyethylene terephthalate (PET) is a widely used synthetic polymer in textile manufacturing, known for its excellent strength, durability, and resistance to moisture and chemicals. Nylon, another popular synthetic fiber, offers superior elasticity, abrasion resistance, and a smooth texture, making it ideal for activewear and hosiery. Both PET and Nylon are thermoplastic polymers derived from petrochemicals, but PET is favored for its dimensional stability and recycling potential, while Nylon excels in stretch and resilience.
Chemical Structure and Composition
Polyethylene terephthalate (PET) is a polyester composed of repeating units of ethylene glycol and terephthalic acid, characterized by ester functional groups that provide good thermal stability and moisture resistance in textiles. Nylon, a polyamide, consists of repeating units linked by amide bonds derived from diamines and dicarboxylic acids, offering higher elasticity and moisture absorption due to its polar amide groups. The chemical structure differences between PET's ester linkages and Nylon's amide linkages significantly influence their mechanical properties, dye affinity, and resistance to environmental factors in textile applications.
Manufacturing Process Comparison
Polyethylene terephthalate (PET) is produced through a polycondensation reaction between terephthalic acid and ethylene glycol, typically involving melt spinning to create fibers used in textiles. Nylon manufacturing involves the polymerization of diamines and dicarboxylic acids or lactams, followed by extrusion and drawing to form strong, elastic fibers. PET's production emphasizes thermal stability and hydrophobicity, whereas nylon's process focuses on achieving superior tensile strength and moisture absorption properties, influencing their distinct performance in textile applications.
Mechanical and Physical Properties
Polyethylene terephthalate (PET) exhibits high tensile strength, excellent abrasion resistance, and low moisture absorption, making it ideal for durable textile applications requiring dimensional stability. Nylon offers superior elasticity, higher impact resistance, and better flexibility, but it absorbs more moisture, potentially affecting weight and drying times. Both fibers demonstrate strong mechanical properties, yet PET's rigidity contrasts with nylon's enhanced resilience and stretchability in textile manufacturing.
Durability and Strength in Textiles
Polyethylene terephthalate (PET) offers excellent durability in textiles due to its high resistance to abrasion, UV light, and environmental degradation, making it ideal for outdoor and sportswear applications. Nylon provides superior tensile strength and elasticity, which enhances its performance in high-stress and stretch-intensive textile applications such as activewear and industrial fabrics. Both fibers exhibit excellent longevity, but PET is more resistant to chemical and moisture exposure, while nylon excels in flexibility and impact resistance.
Moisture Absorption and Comfort
Polyethylene terephthalate (PET) exhibits low moisture absorption, typically around 0.4%, resulting in quicker drying times and reduced clamminess, which enhances comfort in textiles worn during physical activity. Nylon, with a moisture absorption rate of approximately 4%, retains more water, offering better breathability and moisture management for garments requiring softness and stretch. The lower hygroscopic nature of PET makes it ideal for moisture-wicking sportswear, while nylon's higher absorption contributes to a comfortable feel in casual and activewear fabrics.
Environmental Impact and Sustainability
Polyethylene terephthalate (PET) has a lower environmental footprint compared to nylon, primarily due to its higher recyclability and lower greenhouse gas emissions during production. Nylon production emits nitrous oxide, a potent greenhouse gas, and involves significant energy consumption, while PET can be efficiently recycled into new textiles, reducing landfill waste. Sustainable initiatives increasingly favor PET for textile applications due to its closed-loop recycling potential and reduced dependency on fossil fuel inputs relative to nylon.
Cost and Market Availability
Polyethylene terephthalate (PET) is significantly more cost-effective than nylon, with prices generally lower due to its widespread production and use in various textile applications. PET enjoys broader market availability, being extensively used in apparel, packaging, and industrial fabrics, whereas nylon's market is more specialized, often commanding higher prices due to its superior strength and elasticity. The global production scale of PET creates economies of scale that enhance its accessibility and affordability compared to nylon, which is produced in smaller volumes.
Common Applications in the Textile Industry
Polyethylene terephthalate (PET) is widely used in textile manufacturing for its durability, moisture-wicking properties, and resistance to shrinking, making it ideal for activewear, outdoor gear, and upholstery fabrics. Nylon excels in applications requiring high elasticity, abrasion resistance, and strength, commonly found in swimwear, hosiery, and performance apparel. Both fibers are favored for their versatility but serve distinct roles based on their unique physical and chemical properties in textile production.
Summary: Choosing Between PET and Nylon in Textiles
Polyethylene terephthalate (PET) and nylon are both widely used synthetic fibers in textiles, with PET known for its excellent moisture resistance, durability, and recyclability, making it ideal for sustainable and high-performance fabrics. Nylon offers superior elasticity, abrasion resistance, and strength, which are essential for applications requiring stretch and resilience, such as sportswear and technical textiles. The choice between PET and nylon depends on specific textile demands like environmental impact, fabric texture, and functional properties, ensuring optimal performance for the intended use.
Infographic: Polyethylene terephthalate vs Nylon for Textile
 azmater.com
azmater.com