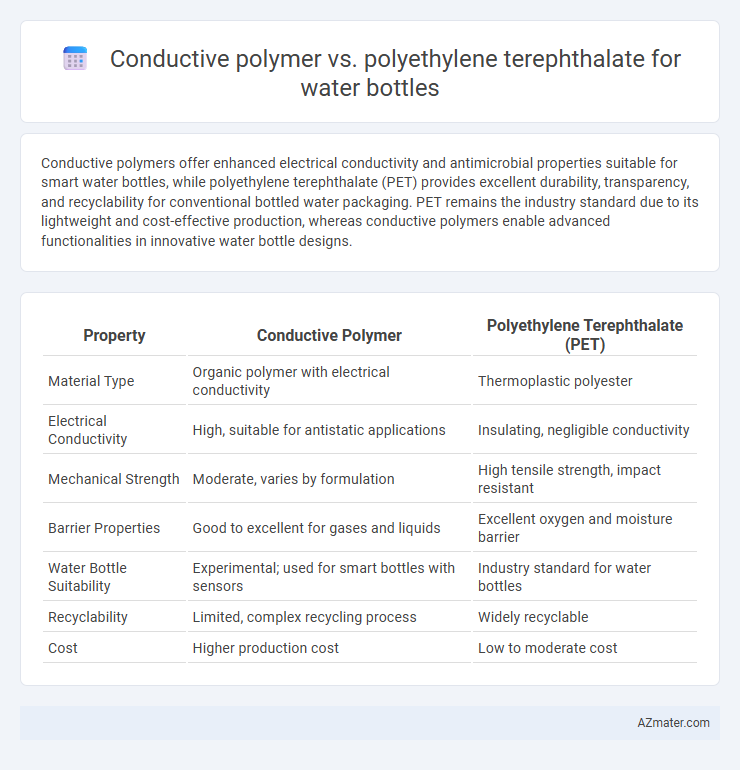
Conductive polymers offer enhanced electrical conductivity and antimicrobial properties suitable for smart water bottles, while polyethylene terephthalate (PET) provides excellent durability, transparency, and recyclability for conventional bottled water packaging. PET remains the industry standard due to its lightweight and cost-effective production, whereas conductive polymers enable advanced functionalities in innovative water bottle designs.
Table of Comparison
| Property | Conductive Polymer | Polyethylene Terephthalate (PET) |
|---|---|---|
| Material Type | Organic polymer with electrical conductivity | Thermoplastic polyester |
| Electrical Conductivity | High, suitable for antistatic applications | Insulating, negligible conductivity |
| Mechanical Strength | Moderate, varies by formulation | High tensile strength, impact resistant |
| Barrier Properties | Good to excellent for gases and liquids | Excellent oxygen and moisture barrier |
| Water Bottle Suitability | Experimental; used for smart bottles with sensors | Industry standard for water bottles |
| Recyclability | Limited, complex recycling process | Widely recyclable |
| Cost | Higher production cost | Low to moderate cost |
Overview of Conductive Polymers and Polyethylene Terephthalate (PET)
Conductive polymers are organic materials that exhibit electrical conductivity, making them suitable for applications requiring flexibility and conductivity, such as sensors and smart packaging. Polyethylene terephthalate (PET) is a widely used thermoplastic polymer known for its strength, transparency, and excellent barrier properties, commonly used in water bottles due to its durability and recyclability. While PET offers robust mechanical performance and chemical resistance for water containment, conductive polymers provide added functionality through electrical conductivity but typically lack the structural strength of PET in bottle applications.
Chemical Structure and Composition Comparison
Conductive polymers, such as polyaniline or polypyrrole, exhibit conjugated double bonds within their backbone, allowing electron mobility and electrical conductivity, whereas polyethylene terephthalate (PET) consists of repeating ester groups derived from terephthalic acid and ethylene glycol, resulting in a non-conductive, thermoplastic structure. Conductive polymers feature heteroatoms like nitrogen or sulfur integrated into their polymer chains, which facilitate redox reactions and charge transport, contrasting with PET's stable aromatic rings and ester linkages that provide durability and chemical resistance. The distinct chemical compositions impact water bottle applications by influencing properties such as antimicrobial activity, recyclability, and mechanical performance, with PET favored for its strength and transparency but lacking the intrinsic conductivity of conductive polymers.
Mechanical Properties: Strength, Flexibility, and Durability
Conductive polymers exhibit moderate strength and enhanced flexibility, making them suitable for applications requiring electrical conductivity alongside mechanical resilience. Polyethylene terephthalate (PET) offers high tensile strength, excellent impact resistance, and superior durability, which are critical for water bottles subjected to repeated use and stress. While conductive polymers provide unique functional properties, PET remains the preferred material for water bottles due to its balanced combination of mechanical robustness and long-term durability.
Electrical Conductivity: Applications in Water Bottles
Conductive polymers offer significant electrical conductivity, enabling applications such as anti-static water bottles and sensors for real-time monitoring of water quality, unlike polyethylene terephthalate (PET), which is an electrical insulator. PET remains the preferred choice for standard water bottles due to its durability, chemical resistance, and low cost but lacks the capability to support integrated electronic functions. The integration of conductive polymers in water bottles enhances smart packaging technologies, providing added functionality like leak detection and contamination alerts.
Barrier Properties: Oxygen and Moisture Resistance
Conductive polymers generally exhibit lower oxygen and moisture barrier properties compared to polyethylene terephthalate (PET), making PET a preferred choice for water bottles requiring high barrier performance. PET provides excellent resistance against oxygen permeation and moisture absorption, ensuring longer shelf life and maintaining water purity. The superior crystallinity and molecular structure of PET significantly reduce gas transmission rates compared to conductive polymers, which often lack the dense polymer matrix needed for effective barrier functionality.
Safety and Toxicity Concerns in Drinking Water Storage
Conductive polymers, often used for specialized applications, may release toxic substances or degrade under prolonged water exposure, raising safety concerns for drinking water storage. Polyethylene terephthalate (PET) is widely favored for water bottles due to its stable chemical structure, low toxicity, and regulatory approval for safe food and beverage contact. Studies confirm PET's resistance to leaching harmful compounds, making it a safer choice compared to conductive polymers in terms of toxicity and water safety.
Environmental Impact: Biodegradability and Recycling
Conductive polymers generally exhibit limited biodegradability and pose challenges in recycling due to their complex chemical structures and incorporation of conductive fillers. Polyethylene terephthalate (PET) is widely recognized for its excellent recyclability through established municipal systems and relatively low environmental impact when recycled properly, although it remains non-biodegradable. The environmental footprint of water bottles can be significantly reduced by prioritizing PET materials with efficient recycling practices over conductive polymers, which currently lack scalable eco-friendly disposal options.
Cost-Effectiveness and Manufacturing Considerations
Conductive polymers generally exhibit higher material costs compared to polyethylene terephthalate (PET) due to specialized synthesis processes and raw material prices, impacting overall water bottle production expenses. PET offers proven cost-effectiveness with established large-scale manufacturing infrastructure, enabling faster production cycles and lower energy consumption, which reduces per-unit costs. Manufacturing considerations favor PET for water bottles, given its excellent mechanical properties, recyclability, and compatibility with injection molding and blow molding technologies, while conductive polymers require more complex extrusion techniques and may face challenges in scalability and consistency.
Practical Applications and Market Trends
Conductive polymers, known for their electrical conductivity and flexibility, are increasingly explored for smart water bottle applications featuring integrated sensors for hydration tracking and temperature monitoring. Polyethylene terephthalate (PET) remains the dominant material in the water bottle market due to its lightweight, durability, recyclability, and cost-effectiveness. Market trends indicate a growing interest in combining PET with conductive polymers to develop multifunctional bottles that meet consumer demand for both sustainability and advanced features.
Future Prospects in Sustainable Water Bottle Materials
Conductive polymers offer innovative opportunities in sustainable water bottle materials by enabling smart functionalities such as contamination detection and self-cleaning surfaces, which polyethylene terephthalate (PET) lacks. PET remains dominant due to its established recyclability and low cost, but its future is challenged by environmental concerns and reliance on fossil fuels. Advancements in bio-based conductive polymers could revolutionize sustainable packaging, combining biodegradability with enhanced functionality for next-generation water bottles.
Infographic: Conductive polymer vs Polyethylene terephthalate for Water bottle
 azmater.com
azmater.com