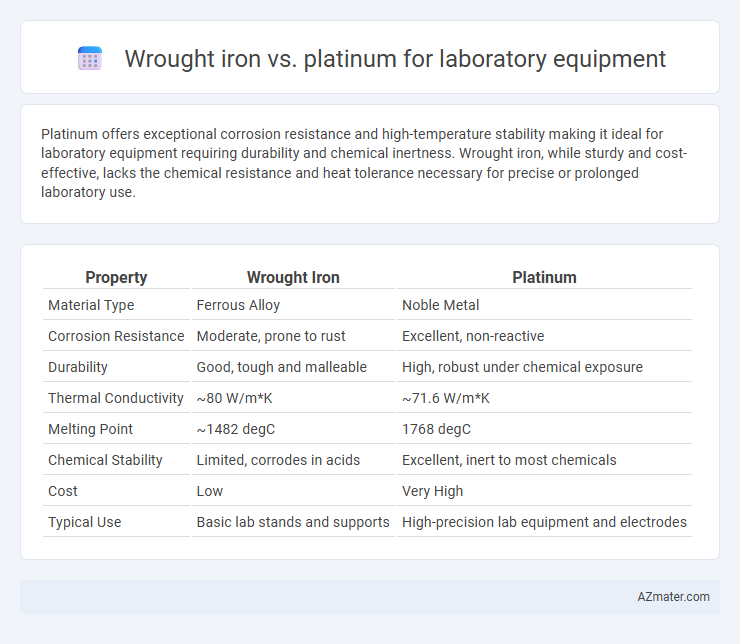
Platinum offers exceptional corrosion resistance and high-temperature stability making it ideal for laboratory equipment requiring durability and chemical inertness. Wrought iron, while sturdy and cost-effective, lacks the chemical resistance and heat tolerance necessary for precise or prolonged laboratory use.
Table of Comparison
| Property | Wrought Iron | Platinum |
|---|---|---|
| Material Type | Ferrous Alloy | Noble Metal |
| Corrosion Resistance | Moderate, prone to rust | Excellent, non-reactive |
| Durability | Good, tough and malleable | High, robust under chemical exposure |
| Thermal Conductivity | ~80 W/m*K | ~71.6 W/m*K |
| Melting Point | ~1482 degC | 1768 degC |
| Chemical Stability | Limited, corrodes in acids | Excellent, inert to most chemicals |
| Cost | Low | Very High |
| Typical Use | Basic lab stands and supports | High-precision lab equipment and electrodes |
Introduction to Laboratory Materials
Wrought iron and platinum are two distinct materials used in laboratory equipment, each with unique properties suited for specific applications. Wrought iron is known for its durability, malleability, and cost-effectiveness, often employed in structural supports and non-reactive components. Platinum, prized for its exceptional corrosion resistance, high melting point, and chemical inertness, is preferred in crucibles, electrodes, and high-temperature reactions where contamination must be minimized.
Overview of Wrought Iron in Laboratory Applications
Wrought iron is valued in laboratory equipment for its durability, corrosion resistance, and ease of fabrication, making it ideal for structural supports and heavy-duty fixtures. Its porous nature allows it to withstand repeated heating and cooling cycles without deforming, which is crucial for maintaining equipment stability. Unlike platinum, wrought iron offers a cost-effective solution for large-scale apparatus requiring mechanical strength rather than chemical inertness.
Platinum in Laboratory Settings: Key Features
Platinum offers exceptional chemical resistance and high melting point, making it ideal for laboratory settings that involve corrosive reagents and extreme temperatures. Unlike wrought iron, platinum does not oxidize or corrode, ensuring long-term durability and minimal contamination during experiments. Its excellent catalytic properties also enhance accuracy and reliability in sensitive chemical analyses.
Physical and Chemical Properties Comparison
Wrought iron exhibits high tensile strength and malleability but is prone to corrosion and oxidation, limiting its chemical resistance in laboratory environments. Platinum offers exceptional corrosion resistance, high melting point (1,768 degC), and excellent chemical inertness, making it ideal for handling reactive substances and high-temperature applications. While wrought iron is economical and durable under mechanical stress, platinum's superior chemical stability and non-reactivity offer unmatched performance in precise and contamination-sensitive lab equipment.
Resistance to Corrosion and Chemical Reactivity
Platinum exhibits superior resistance to corrosion and chemical reactivity compared to wrought iron, making it ideal for laboratory equipment exposed to aggressive chemicals and high temperatures. Wrought iron is prone to rust and corrosion when in contact with acids or moisture, limiting its durability in chemical environments. The inert nature of platinum ensures long-term stability and minimal contamination in sensitive laboratory applications.
Thermal Stability and Heat Resistance
Platinum exhibits superior thermal stability and heat resistance compared to wrought iron, maintaining structural integrity at temperatures exceeding 1,700degC, which is critical for high-temperature laboratory applications. Wrought iron, with a melting point around 1,500degC, is prone to oxidation and structural degradation under prolonged heat exposure, limiting its use in precise thermal processes. The exceptional corrosion resistance and minimal thermal expansion of platinum ensure consistent performance in laboratory equipment subjected to repeated heating and cooling cycles.
Cost Analysis: Wrought Iron vs Platinum
Wrought iron offers a significantly lower cost compared to platinum, making it a budget-friendly option for laboratory equipment where budget constraints are critical. Platinum's high price is justified by its exceptional chemical resistance, durability, and corrosion resistance, which can reduce long-term replacement and maintenance expenses in demanding laboratory environments. Cost analysis should weigh initial investment against lifespan and performance requirements, with wrought iron suitable for less corrosive applications and platinum preferred for high-purity, contamination-sensitive settings.
Durability and Longevity in Laboratory Use
Wrought iron offers high durability with exceptional resistance to mechanical wear, making it suitable for heavy-duty laboratory equipment, but it is prone to corrosion and requires regular maintenance. Platinum, although more expensive, provides superior longevity due to its excellent resistance to corrosion, chemical inertness, and ability to withstand extreme temperatures without degradation. For long-term laboratory use, platinum's durability in harsh chemical environments significantly surpasses wrought iron, ensuring consistent performance and minimal equipment replacement.
Typical Applications and Suitability
Wrought iron is commonly used in laboratory equipment requiring structural support and durability, such as stands, clamps, and framework, due to its toughness and cost-effectiveness. Platinum is favored for high-precision applications involving chemical resistance and high-temperature environments, including crucibles, electrodes, and catalytic surfaces, because of its inertness and ability to withstand corrosion. Suitability depends on the laboratory task; wrought iron excels in mechanical stability while platinum is ideal for advanced chemical and thermal processes.
Conclusion: Selecting the Right Material for Laboratory Equipment
Wrought iron offers durability and cost-effectiveness but lacks the corrosion resistance and inert properties essential for precise laboratory work, making it suitable mainly for structural components. Platinum, with its exceptional chemical inertness, high melting point, and resistance to oxidation, is ideal for critical laboratory applications involving high temperatures and reactive chemicals. Selecting the right material depends on the specific laboratory requirements, balancing budget constraints with the need for chemical stability and durability.
Infographic: Wrought iron vs Platinum for Laboratory equipment
 azmater.com
azmater.com