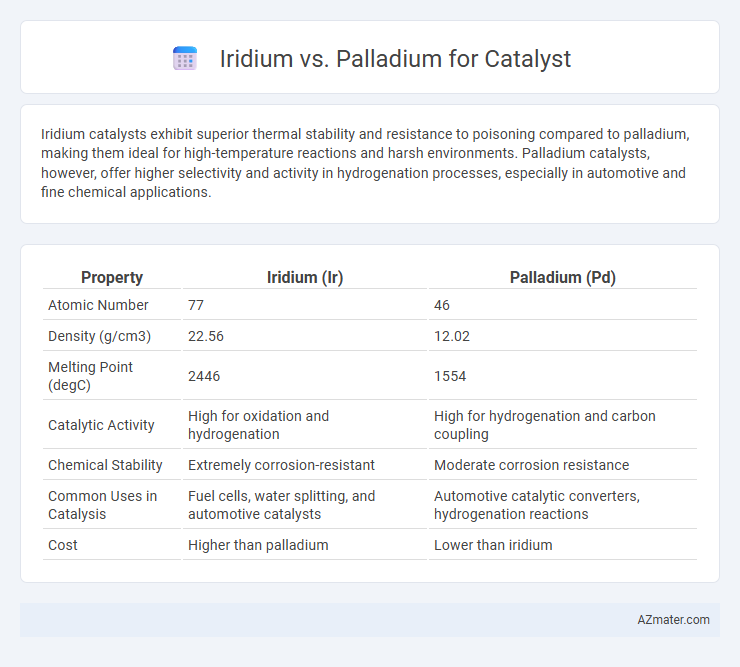
Iridium catalysts exhibit superior thermal stability and resistance to poisoning compared to palladium, making them ideal for high-temperature reactions and harsh environments. Palladium catalysts, however, offer higher selectivity and activity in hydrogenation processes, especially in automotive and fine chemical applications.
Table of Comparison
| Property | Iridium (Ir) | Palladium (Pd) |
|---|---|---|
| Atomic Number | 77 | 46 |
| Density (g/cm3) | 22.56 | 12.02 |
| Melting Point (degC) | 2446 | 1554 |
| Catalytic Activity | High for oxidation and hydrogenation | High for hydrogenation and carbon coupling |
| Chemical Stability | Extremely corrosion-resistant | Moderate corrosion resistance |
| Common Uses in Catalysis | Fuel cells, water splitting, and automotive catalysts | Automotive catalytic converters, hydrogenation reactions |
| Cost | Higher than palladium | Lower than iridium |
Introduction to Iridium and Palladium Catalysts
Iridium and palladium catalysts are widely utilized in chemical reactions due to their exceptional ability to facilitate bond formation and cleavage. Iridium catalysts excel in hydrogenation and C-H activation processes, offering high selectivity and stability under harsh conditions, while palladium catalysts are renowned for their efficiency in cross-coupling reactions such as Suzuki and Heck reactions, enabling the formation of carbon-carbon bonds. The distinct electronic properties and coordination chemistry of iridium and palladium make them indispensable in synthetic organic chemistry and industrial applications.
Chemical Properties Comparison
Iridium exhibits exceptional chemical stability and resistance to oxidation, making it highly effective for catalytic processes involving harsh reaction conditions. Palladium demonstrates remarkable hydrogen absorption and affinity, facilitating selective hydrogenation and carbon-carbon bond formation reactions. Both metals serve crucial catalytic roles; however, iridium excels in high-temperature and oxidative environments, while palladium is preferred for reactions requiring rapid hydrogen uptake and transfer.
Catalytic Mechanisms: Iridium vs Palladium
Iridium catalysts exhibit high efficiency in hydrogenation and hydroformylation reactions due to their strong oxidative addition and reductive elimination capabilities, enabling precise control over stereochemistry. Palladium catalysts excel in cross-coupling reactions such as Suzuki and Heck due to their facile formation of Pd(0)/Pd(II) cycles, enhancing C-C bond formation with broad substrate scope. The differing electronic structures of iridium and palladium influence their catalytic cycles, where iridium favors stable, high-valent intermediates, while palladium operates through more labile, lower-valent species, impacting reaction rates and selectivities.
Applications in Industrial Processes
Iridium and palladium serve distinct roles in industrial catalyst applications, with iridium excelling in high-temperature reactions such as water electrolysis and oxidative processes due to its exceptional corrosion resistance and stability. Palladium dominates in hydrogenation and carbon-carbon coupling reactions, widely used in petrochemical refining, pharmaceuticals, and automotive catalytic converters because of its superior hydrogen absorption and catalytic activity. Both metals are critical for advancing green chemistry and sustainable industrial manufacturing, with iridium favored for electrochemical catalysts and palladium preferred for organic synthesis and pollution control.
Efficiency and Selectivity in Reactions
Iridium catalysts demonstrate higher efficiency in hydrogenation and C-H activation reactions due to their ability to stabilize diverse oxidation states, leading to enhanced turnover frequencies compared to palladium. Palladium catalysts excel in selective cross-coupling reactions such as Suzuki and Heck, offering superior selectivity by minimizing side reactions and promoting precise bond formation. The choice between iridium and palladium catalysts depends on reaction specificity, with iridium favored for oxidation and hydrogenation efficiency, while palladium is preferred for coupling reaction selectivity.
Cost and Availability of Each Metal
Iridium is significantly more expensive than palladium, driven by its rarity in the Earth's crust and limited mining sources, with prices often exceeding $6,000 per ounce compared to palladium's $2,000-$3,000 range. Palladium benefits from higher availability due to more widespread mining, particularly in Russia and South Africa, resulting in greater market supply and lower volatility in cost. The cost-effectiveness and relative abundance of palladium make it a more practical choice for catalyst applications, whereas iridium's scarcity and price restrict its usage despite superior catalytic performance in certain reactions.
Environmental Impact and Sustainability
Iridium catalysts exhibit higher durability and resistance to corrosion, enabling longer lifespans and reduced material consumption compared to palladium catalysts. Palladium, while effective in catalytic converters for reducing vehicle emissions, requires intensive mining processes that generate significant environmental pollution and habitat disruption. Iridium's scarcity poses sustainability challenges, but its superior catalytic efficiency and recyclability contribute to lower overall ecological footprints in catalytic applications.
Recent Advances in Catalyst Technology
Recent advances in catalyst technology highlight the unique roles of iridium and palladium in catalytic processes, with iridium excelling in water-splitting reactions due to its high stability and oxygen evolution efficiency. Palladium remains a preferred choice in hydrogenation and carbon-carbon coupling reactions owing to its superior selectivity and activity under mild conditions. Innovations in nanoparticle synthesis and alloy formation have further enhanced the catalytic performance and durability of both metals, driving progress in clean energy and fine chemical production.
Challenges and Limitations
Iridium catalysts face challenges including high cost, limited availability, and susceptibility to deactivation under harsh reaction conditions, which constrain their industrial scalability. Palladium catalysts often encounter issues like metal leaching, sensitivity to sulfur poisoning, and lower stability in oxidative environments that reduce their longevity. Both metals require careful optimization to balance activity, selectivity, and durability in catalytic applications, impacting overall performance and economic feasibility.
Future Trends in Catalysis Research
Iridium and palladium play crucial roles in catalysis research, with iridium gaining prominence due to its exceptional stability and efficiency in high-value oxidation reactions and water splitting applications. Palladium remains widely used for cross-coupling and hydrogenation reactions, but research increasingly explores iridium-based catalysts for sustainable energy and green chemistry solutions. Future trends emphasize the development of iridium-palladium hybrid catalysts to combine their unique properties, enhancing catalytic performance and durability in industrial and environmental applications.
Infographic: Iridium vs Palladium for Catalyst
 azmater.com
azmater.com