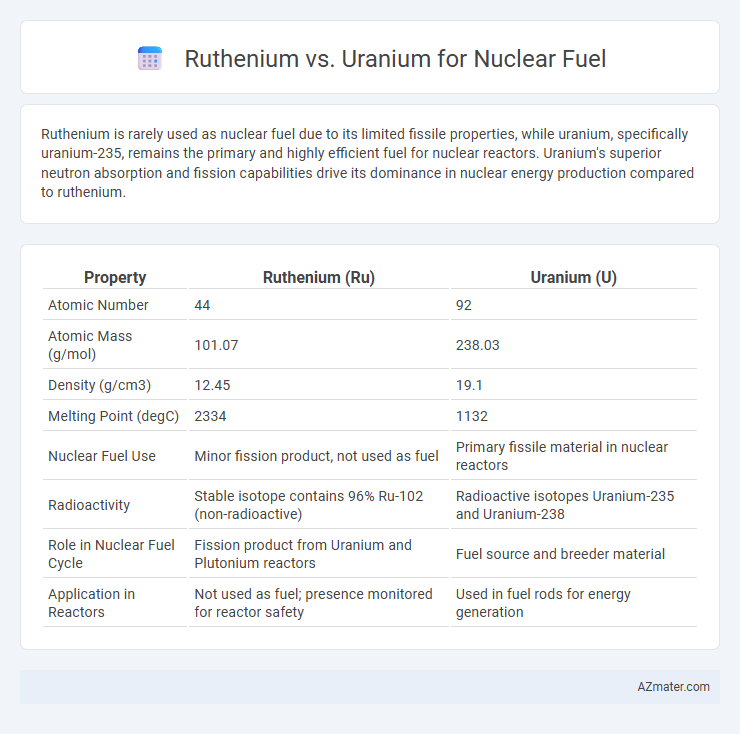
Ruthenium is rarely used as nuclear fuel due to its limited fissile properties, while uranium, specifically uranium-235, remains the primary and highly efficient fuel for nuclear reactors. Uranium's superior neutron absorption and fission capabilities drive its dominance in nuclear energy production compared to ruthenium.
Table of Comparison
| Property | Ruthenium (Ru) | Uranium (U) |
|---|---|---|
| Atomic Number | 44 | 92 |
| Atomic Mass (g/mol) | 101.07 | 238.03 |
| Density (g/cm3) | 12.45 | 19.1 |
| Melting Point (degC) | 2334 | 1132 |
| Nuclear Fuel Use | Minor fission product, not used as fuel | Primary fissile material in nuclear reactors |
| Radioactivity | Stable isotope contains 96% Ru-102 (non-radioactive) | Radioactive isotopes Uranium-235 and Uranium-238 |
| Role in Nuclear Fuel Cycle | Fission product from Uranium and Plutonium reactors | Fuel source and breeder material |
| Application in Reactors | Not used as fuel; presence monitored for reactor safety | Used in fuel rods for energy generation |
Introduction: Ruthenium vs Uranium in Nuclear Fuel
Ruthenium and uranium serve distinct roles in nuclear fuel applications, with uranium being the primary fissile material used in most nuclear reactors due to its ability to sustain a chain reaction. Ruthenium, a platinum-group metal, is not utilized as fuel but is recognized as a fission product and also explored for its catalytic properties in nuclear fuel reprocessing. Understanding their differences is crucial for optimizing nuclear fuel cycles and improving reactor efficiency.
Elemental Properties Compared
Ruthenium and uranium differ significantly in atomic number, with ruthenium at 44 and uranium at 92, impacting their nuclear properties and applications. Uranium's high atomic mass and fissile isotopes, such as U-235, make it suitable for nuclear fuel in reactors, while ruthenium's lower atomic mass and stable isotopes limit its use in energy generation. The density of uranium (approximately 19.1 g/cm3) surpasses ruthenium's (about 12.4 g/cm3), contributing to uranium's ability to sustain nuclear fission, contrasted with ruthenium's primary use in electronics and catalysts due to its corrosion resistance and stable physical properties.
Abundance and Availability
Ruthenium is a rare transition metal with Earth's crust abundance of approximately 0.001 ppm, making it significantly scarcer than uranium, which is found at about 2.7 ppm. Uranium's widespread availability in continental rocks and established mining infrastructure ensures a more reliable supply for nuclear fuel production. Limited abundance and high extraction costs constrain ruthenium's feasibility as a nuclear fuel compared to uranium's strategic role in current nuclear energy systems.
Nuclear Fission Characteristics
Ruthenium exhibits limited nuclear fission characteristics, primarily serving as a fission product rather than a fuel, with low neutron absorption cross-section and negligible fissile properties. Uranium, particularly isotopes U-235 and U-238, demonstrates significant fission capabilities due to high neutron absorption cross-sections and the ability to sustain chain reactions essential for nuclear reactors. The fissile nature of uranium enables efficient energy release through controlled fission, whereas ruthenium's role is mostly passive, influencing reactor behavior through its radioactive decay products.
Energy Output Potential
Uranium, specifically U-235, is the primary fuel for nuclear reactors due to its high energy output potential, releasing approximately 24 million electron volts (MeV) per fission event. Ruthenium, a byproduct of nuclear fission, is not used as a fuel but is valued for its stability and corrosion resistance in reactor components rather than energy generation. The significantly greater fissile material density and energy release rate of uranium make it the preferred choice for efficient and sustained nuclear fuel applications.
Radiological Safety and Handling
Ruthenium and uranium differ significantly in radiological safety and handling for nuclear fuel applications. Uranium, a primary fuel in nuclear reactors, poses substantial radiological risks due to its high radioactivity and long half-life, necessitating stringent shielding, containment, and specialized handling protocols to protect workers and the environment. Ruthenium, primarily a fission product rather than a fuel, exhibits lower radiological hazards but requires careful management due to its chemical toxicity and potential release during fuel reprocessing, with safety measures focusing on preventing environmental contamination and operator exposure.
Reactor Compatibility and Applications
Ruthenium and uranium exhibit stark differences in nuclear fuel applications, with uranium being the primary fuel due to its fissile isotopes such as U-235, which sustain chain reactions in most nuclear reactors. Ruthenium, a platinum-group metal, is not used as a primary fuel but appears in small quantities as a fission product during uranium fuel burnup, influencing fuel behavior and reactor safety assessments. Reactor compatibility favors uranium's well-established nuclear properties and existing fuel fabrication infrastructure, whereas ruthenium's role lies in waste management and material science rather than direct fuel substitution.
Environmental Impact and Sustainability
Ruthenium, often used as a nuclear fuel additive, exhibits lower radiotoxicity and shorter half-life isotopes compared to uranium, resulting in reduced long-term environmental contamination. Uranium, the primary nuclear fuel, generates significant radioactive waste with long-lived isotopes like U-238 and U-235, posing challenges for sustainable waste management and environmental protection. Ruthenium's role in advanced fuel cycles enhances fuel efficiency and waste mitigation, contributing to more sustainable nuclear energy solutions.
Economic Considerations
Ruthenium offers limited economic viability as nuclear fuel due to its rarity and higher cost compared to uranium, which remains the predominant choice thanks to its abundant availability and established supply chains. Uranium's well-developed mining infrastructure and existing enrichment technologies result in lower fuel production costs and more predictable market prices. Investing in uranium fuel cycles ensures better economic efficiency and scalability for nuclear power generation compared to the niche application potentials of ruthenium.
Future Prospects in Nuclear Fuel Technology
Ruthenium, primarily a fission product in nuclear reactors, is being researched for its potential as a durable catalyst in advanced nuclear fuel cycles, while uranium remains the dominant fissile material due to its high availability and established fuel fabrication technologies. Future nuclear fuel technology aims to enhance uranium's utilization through innovations like accident-tolerant fuels and fast reactors, potentially integrating ruthenium to improve corrosion resistance and fuel efficiency. The evolving synergy between uranium-based fuels and ruthenium's catalytic properties could drive breakthroughs in reactor performance and sustainable nuclear energy production.
Infographic: Ruthenium vs Uranium for Nuclear Fuel
 azmater.com
azmater.com