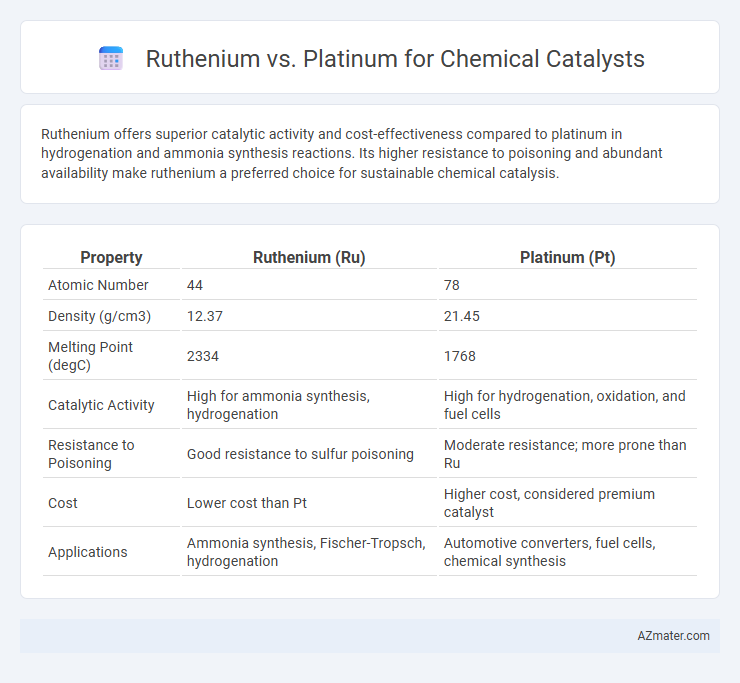
Ruthenium offers superior catalytic activity and cost-effectiveness compared to platinum in hydrogenation and ammonia synthesis reactions. Its higher resistance to poisoning and abundant availability make ruthenium a preferred choice for sustainable chemical catalysis.
Table of Comparison
| Property | Ruthenium (Ru) | Platinum (Pt) |
|---|---|---|
| Atomic Number | 44 | 78 |
| Density (g/cm3) | 12.37 | 21.45 |
| Melting Point (degC) | 2334 | 1768 |
| Catalytic Activity | High for ammonia synthesis, hydrogenation | High for hydrogenation, oxidation, and fuel cells |
| Resistance to Poisoning | Good resistance to sulfur poisoning | Moderate resistance; more prone than Ru |
| Cost | Lower cost than Pt | Higher cost, considered premium catalyst |
| Applications | Ammonia synthesis, Fischer-Tropsch, hydrogenation | Automotive converters, fuel cells, chemical synthesis |
Introduction to Ruthenium and Platinum Catalysts
Ruthenium catalysts exhibit exceptional activity in hydrogenation, ammonia synthesis, and olefin metathesis, thanks to their unique electronic properties and ability to form diverse coordination complexes. Platinum catalysts are highly valued for their efficiency in oxidation reactions, fuel cells, and automotive catalytic converters, offering excellent stability and resistance to poisoning. Both metals play crucial roles in heterogeneous and homogeneous catalysis, but ruthenium often outperforms platinum in specific processes due to its superior selectivity and cost-effectiveness.
Chemical Properties Comparison
Ruthenium exhibits superior catalytic activity in hydrogenation reactions due to its ability to adsorb hydrogen molecules more effectively than platinum. Platinum offers greater stability and resistance to poisoning, making it ideal for oxidation catalysts in fuel cells and automotive emissions control. Both metals exhibit variable oxidation states, but ruthenium's multiple valence states enable versatile catalytic cycles, while platinum's stable +2 and +4 states support selective catalytic processes.
Catalytic Performance in Key Reactions
Ruthenium exhibits superior catalytic performance in ammonia synthesis and hydrogenation reactions due to its high activity and ability to operate under milder conditions compared to platinum. Platinum, while renowned for its excellent stability and selectivity in oxidation reactions such as in fuel cells and catalytic converters, often requires higher temperatures to achieve comparable activity. The choice between ruthenium and platinum catalysts depends on specific reaction pathways, substrate types, and desired product yields, with ruthenium favored for nitrogen fixation and platinum preferred in automotive exhaust treatment.
Cost and Economic Viability
Ruthenium offers a more cost-effective alternative to platinum as a chemical catalyst, with ruthenium prices generally lower than platinum by 30-50%, improving economic viability for large-scale industrial applications. Ruthenium exhibits high catalytic efficiency in ammonia synthesis and hydrogenation reactions, enabling reduced catalyst loading and operational costs compared to platinum. While platinum remains valuable for its exceptional durability and resistance to poisoning, ruthenium's lower cost and comparable catalytic performance make it an attractive option for cost-sensitive catalytic processes.
Stability and Longevity in Catalytic Cycles
Ruthenium exhibits superior stability in harsh catalytic cycles, maintaining active sites under oxidative and reductive conditions better than platinum. Platinum catalysts, while highly effective, often suffer from sintering and deactivation more quickly during extended use. The longevity of ruthenium-based catalysts makes them preferable in industrial processes requiring durable, long-lasting catalysts, such as ammonia synthesis and hydrogenation reactions.
Environmental Impact and Sustainability
Ruthenium offers a more sustainable alternative to platinum in chemical catalysis due to its lower cost, higher abundance, and comparable catalytic efficiency in key reactions such as hydrogenation and ammonia synthesis. Its reduced environmental footprint stems from less energy-intensive extraction processes and greater recyclability, which helps minimize resource depletion and waste generation. Platinum, while highly effective, is scarcer and associated with more environmentally damaging mining practices, making ruthenium increasingly preferred for eco-friendly catalytic applications.
Applications in Industrial Processes
Ruthenium exhibits exceptional catalytic properties in ammonia synthesis and Fischer-Tropsch processes, making it highly efficient for converting synthesis gas into valuable hydrocarbons. Platinum, renowned for its durability and broad applicability, is widely used in automotive catalytic converters and fuel cell technologies to facilitate oxidation and hydrogenation reactions. Industrial processes favor ruthenium for high selectivity in olefin metathesis, while platinum dominates in hydrogenation reactions and oxidation of volatile organic compounds due to its superior stability under harsh conditions.
Selectivity and Efficiency Differences
Ruthenium catalysts demonstrate higher selectivity in hydrogenation reactions compared to platinum, particularly in reducing aromatic rings without over-hydrogenation. Platinum catalysts often exhibit superior efficiency in general hydrogenation due to their broader activity scope but can suffer from lower selectivity, leading to undesired by-products. The distinct electronic properties of ruthenium enable precise control over reaction pathways, enhancing product specificity, while platinum's versatility supports faster reaction rates across varied substrates.
Recent Advancements in Ruthenium and Platinum Catalysis
Recent advancements in ruthenium catalysis have demonstrated its superior efficiency in hydrogenation and oxidation reactions due to its versatile oxidation states and ability to activate small molecules at lower temperatures. Platinum catalysis continues to dominate in automotive catalytic converters and fuel cells, with cutting-edge research enhancing its durability and resistance to poisoning through nanoparticle engineering and alloying with non-precious metals. Innovations in both metals focus on improving selectivity and turnover frequency, enabling sustainable chemical processes with lower environmental impact.
Future Prospects and Emerging Trends
Ruthenium exhibits promising future prospects in chemical catalysis due to its superior catalytic activity and lower cost compared to platinum, particularly in hydrogenation and ammonia synthesis. Emerging trends highlight ruthenium-based nanostructures and bimetallic alloys that enhance selectivity and durability under harsh reaction conditions. Ongoing research emphasizes sustainable catalyst design leveraging ruthenium's unique electronic properties to improve efficiency in energy conversion and green chemistry applications.
Infographic: Ruthenium vs Platinum for Chemical Catalyst
 azmater.com
azmater.com