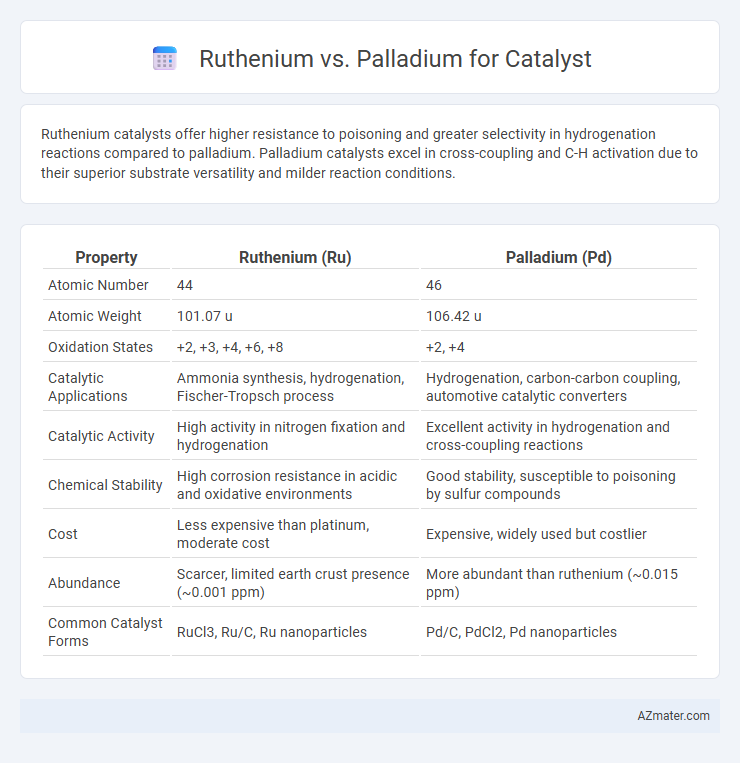
Ruthenium catalysts offer higher resistance to poisoning and greater selectivity in hydrogenation reactions compared to palladium. Palladium catalysts excel in cross-coupling and C-H activation due to their superior substrate versatility and milder reaction conditions.
Table of Comparison
| Property | Ruthenium (Ru) | Palladium (Pd) |
|---|---|---|
| Atomic Number | 44 | 46 |
| Atomic Weight | 101.07 u | 106.42 u |
| Oxidation States | +2, +3, +4, +6, +8 | +2, +4 |
| Catalytic Applications | Ammonia synthesis, hydrogenation, Fischer-Tropsch process | Hydrogenation, carbon-carbon coupling, automotive catalytic converters |
| Catalytic Activity | High activity in nitrogen fixation and hydrogenation | Excellent activity in hydrogenation and cross-coupling reactions |
| Chemical Stability | High corrosion resistance in acidic and oxidative environments | Good stability, susceptible to poisoning by sulfur compounds |
| Cost | Less expensive than platinum, moderate cost | Expensive, widely used but costlier |
| Abundance | Scarcer, limited earth crust presence (~0.001 ppm) | More abundant than ruthenium (~0.015 ppm) |
| Common Catalyst Forms | RuCl3, Ru/C, Ru nanoparticles | Pd/C, PdCl2, Pd nanoparticles |
Introduction to Ruthenium and Palladium Catalysts
Ruthenium and palladium are transition metals extensively used as catalysts in chemical reactions due to their unique electronic structures and surface properties. Ruthenium catalysts are renowned for their high activity in hydrogenation, ammonia synthesis, and Fischer-Tropsch processes, offering excellent selectivity and stability under harsh reaction conditions. Palladium catalysts excel in cross-coupling reactions, hydrogenation of alkenes, and carbon-carbon bond formation, making them vital in pharmaceutical synthesis and fine chemical production.
Chemical Properties and Reactivity
Ruthenium exhibits higher catalytic activity in hydrogenation reactions due to its ability to adsorb hydrogen efficiently, while palladium is renowned for its exceptional selectivity in coupling reactions such as Suzuki and Heck cross-couplings. Ruthenium's chemical properties include multiple oxidation states (+2, +3, +4, +6, +8), facilitating versatile redox behavior, whereas palladium primarily exists in the +0 and +2 states, making it more stable under mild reaction conditions. The difference in electronic configuration and surface chemistry results in ruthenium being more reactive towards small molecules like CO and O2, compared to palladium, which excels in activating C-C and C-H bonds in organic substrates.
Availability and Cost Comparison
Ruthenium and palladium are both precious metals used as catalysts, but palladium is significantly more abundant and widely sourced, making it generally more accessible and affordable in industrial applications. Ruthenium, rarer and often obtained as a byproduct of platinum and nickel mining, has a higher market price due to its limited availability and complex extraction process. Cost comparison reveals palladium typically commands a lower price per ounce, which drives its preference in large-scale catalytic converters and hydrogenation reactions where cost-efficiency is crucial.
Common Applications in Catalysis
Ruthenium is widely used in ammonia synthesis and Fischer-Tropsch reactions due to its high activity for nitrogen and carbon monoxide activation. Palladium plays a crucial role in cross-coupling reactions, hydrogenation, and automotive catalytic converters, valued for its efficiency in carbon-carbon bond formation and hydrogen absorption. Both metals exhibit distinct catalytic properties tailored to specific chemical transformations in industrial and environmental processes.
Activity and Selectivity in Key Reactions
Ruthenium exhibits superior catalytic activity in hydrogenation and ammonia synthesis due to its ability to activate hydrogen molecules efficiently, while palladium excels in selective hydrogenation and carbon-carbon coupling reactions owing to its favorable adsorption properties. Ruthenium catalysts often demonstrate higher turnover frequencies, particularly in Fischer-Tropsch synthesis, whereas palladium catalysts provide enhanced selectivity in cross-coupling reactions such as Suzuki-Miyaura and Heck reactions. The inherent electronic structure of ruthenium allows for stronger metal-support interactions, improving activity, while palladium's surface chemistry promotes precise control over product distribution.
Environmental Impact and Sustainability
Ruthenium catalysts offer higher efficiency in hydrogenation reactions, reducing energy consumption and greenhouse gas emissions compared to palladium catalysts. Palladium, while effective, often requires higher loadings and regeneration processes that can increase environmental footprint. The sustainable use of ruthenium focuses on its longer lifespan and lower demand, contributing to reduced resource depletion and waste in catalytic applications.
Stability and Lifespan of Catalysts
Ruthenium catalysts exhibit superior thermal stability compared to palladium, maintaining activity under harsher reaction conditions and extending catalyst lifespan. Palladium catalysts often face deactivation due to sintering and poisoning, resulting in shorter operational periods in industrial processes. The enhanced durability of ruthenium makes it preferable for high-temperature applications requiring prolonged catalyst stability.
Industrial Case Studies: Ruthenium vs Palladium
Ruthenium catalysts demonstrate superior performance in ammonia synthesis and Fischer-Tropsch processes due to their higher activity and resistance to poisoning compared to palladium. Industrial case studies reveal that palladium catalysts, commonly used in hydrogenation reactions, offer excellent selectivity but may suffer from deactivation under harsh conditions. Ruthenium's robustness and cost-effectiveness have led to increased adoption in large-scale catalytic applications, especially where durability and efficiency are critical.
Recent Advances in Catalyst Design
Recent advances in catalyst design highlight Ruthenium's superior performance in selective hydrogenation and ammonia synthesis due to its unique electronic properties and strong metal-support interactions compared to Palladium. Ruthenium-based catalysts exhibit enhanced stability and higher turnover frequencies in various organic transformations, benefiting from tailored nanostructures and alloy compositions. In contrast, Palladium remains prominent in carbon-carbon coupling reactions but faces challenges in thermal stability and resistance to poisoning, driving research towards hybrid catalyst systems integrating both metals for optimized reactivity.
Future Trends and Research Directions
Ruthenium catalysts show increasing promise due to their exceptional activity in hydrogenation and nitrogen fixation, offering sustainable alternatives to palladium in green chemistry applications. Research trends focus on enhancing ruthenium's selectivity and stability through nanostructuring and alloy formation to lower costs and improve catalytic efficiency. Emerging studies integrate machine learning for catalyst design, accelerating discovery of ruthenium-based materials outperforming traditional palladium catalysts in energy conversion and chemical synthesis.
Infographic: Ruthenium vs Palladium for Catalyst
 azmater.com
azmater.com