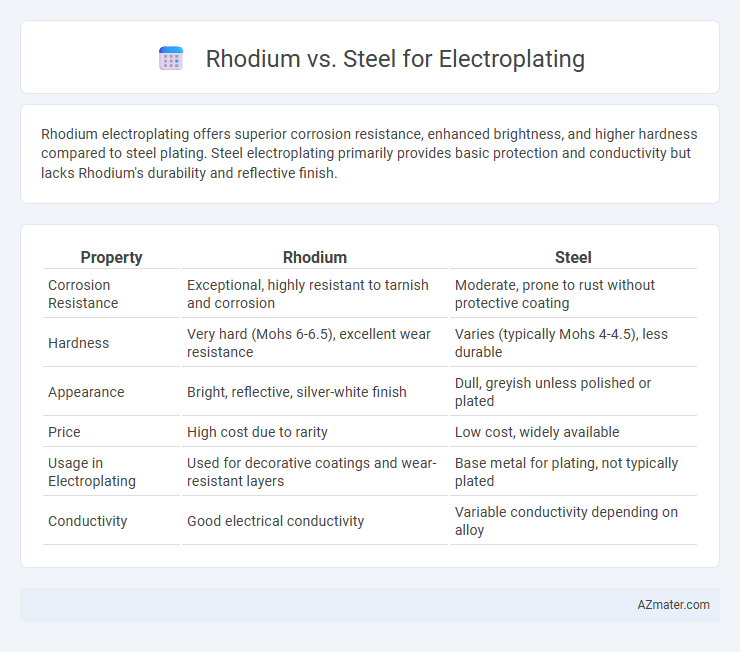
Rhodium electroplating offers superior corrosion resistance, enhanced brightness, and higher hardness compared to steel plating. Steel electroplating primarily provides basic protection and conductivity but lacks Rhodium's durability and reflective finish.
Table of Comparison
| Property | Rhodium | Steel |
|---|---|---|
| Corrosion Resistance | Exceptional, highly resistant to tarnish and corrosion | Moderate, prone to rust without protective coating |
| Hardness | Very hard (Mohs 6-6.5), excellent wear resistance | Varies (typically Mohs 4-4.5), less durable |
| Appearance | Bright, reflective, silver-white finish | Dull, greyish unless polished or plated |
| Price | High cost due to rarity | Low cost, widely available |
| Usage in Electroplating | Used for decorative coatings and wear-resistant layers | Base metal for plating, not typically plated |
| Conductivity | Good electrical conductivity | Variable conductivity depending on alloy |
Introduction to Electroplating Materials
Rhodium and steel serve distinct roles in electroplating, with rhodium prized for its exceptional corrosion resistance, hardness, and brilliant reflective finish, making it ideal for jewelry and automotive trim. Steel, often used as a substrate, requires electroplating to enhance surface properties like durability and rust resistance, leveraging coatings such as nickel or chromium. Electroplating materials must be selected based on factors like conductivity, adhesion, and environmental resistance to ensure optimal performance and longevity.
Overview of Rhodium and Steel
Rhodium, a rare and precious member of the platinum group metals, is highly valued for its exceptional corrosion resistance, brilliant reflective properties, and hypoallergenic characteristics, making it ideal for high-end electroplating applications. Steel, composed primarily of iron with varying amounts of carbon and other elements, offers durability, strength, and affordability, commonly used as a base metal that benefits from rhodium electroplating to enhance surface hardness and aesthetic appeal. The electroplating of rhodium onto steel combines the structural integrity of steel with the superior protective and decorative qualities of rhodium, optimizing performance in jewelry, automotive, and electronic industries.
Chemical Properties: Rhodium vs Steel
Rhodium exhibits exceptional chemical resistance due to its inertness and high corrosion resistance, while steel, primarily composed of iron, is prone to oxidation and rust without protective coatings. Rhodium's hardness and resistance to acids and tarnishing make it ideal for electroplating applications requiring durable, bright finishes. In contrast, steel's reactive surface often necessitates plating to improve its chemical stability and prevent corrosion.
Electroplating Process Differences
Rhodium electroplating involves a more complex process with shorter plating baths, typically using a rhodium sulfate solution to achieve a bright, highly reflective finish. Steel electroplating usually employs nickel or chromium as intermediate layers to enhance adhesion and corrosion resistance before the final plating. The electrochemical properties of rhodium require careful control of current density and temperature, contrasting with the more robust and versatile electroplating conditions suitable for steel substrates.
Durability and Corrosion Resistance
Rhodium electroplating offers superior durability and corrosion resistance compared to steel, making it ideal for high-wear applications and jewelry. Its exceptional hardness and resistance to oxidation prevent tarnishing and extend the lifespan of coated items. Steel, while strong and cost-effective, is more prone to rust and corrosion without protective plating, reducing its long-term durability in corrosive environments.
Aesthetic Outcomes and Finish
Rhodium electroplating provides a brilliant, mirror-like finish with exceptional brightness and high reflectivity, making it a preferred choice for enhancing jewelry and fine watch surfaces. Steel electroplating, while durable and corrosion-resistant, often results in a more utilitarian and less lustrous finish compared to rhodium's superior gloss and resistance to tarnishing. The aesthetic appeal of rhodium plating remains unmatched due to its ability to maintain a long-lasting, vivid white sheen that enhances the overall visual impact of plated items.
Cost and Availability Comparison
Rhodium plating offers superior corrosion resistance and a brilliant finish but comes at a significantly higher cost due to its rarity, with prices often exceeding $10,000 per ounce. Steel plating, typically involving nickel or chrome layers, is more economical and readily available, making it a preferred choice for large-scale industrial applications. The limited global supply of rhodium leads to price volatility, whereas steel materials maintain stable costs, influencing manufacturers' decisions based on budget and production volume.
Environmental Impact and Sustainability
Rhodium electroplating offers superior corrosion resistance and a highly reflective finish but relies on mining processes with significant environmental footprints and limited recyclability, raising sustainability concerns. Steel electroplating, commonly involving zinc or nickel coatings, uses more abundant materials with established recycling systems, reducing environmental impact through circular economy practices. Sustainable electroplating prioritizes minimizing hazardous waste, recovering metals, and adopting greener processes, with steel plating generally presenting lower ecological risks than rhodium due to resource scarcity and toxic byproducts associated with rhodium extraction.
Typical Industrial and Commercial Applications
Rhodium electroplating is widely used in high-end jewelry and automotive trim for its exceptional corrosion resistance, reflective brightness, and ability to prevent tarnishing, making it ideal for luxury and decorative finishes. Steel electroplating, particularly with nickel or zinc layers, is common in industrial machinery, construction tools, and consumer appliances due to its cost-effectiveness, enhanced wear resistance, and improved substrate hardness. Both materials serve crucial roles in electroplating: rhodium offers superior aesthetic and anti-corrosion qualities for premium applications, while steel coatings provide functional durability in heavy-duty commercial environments.
Choosing the Right Material for Electroplating
Rhodium offers exceptional corrosion resistance and a brilliant, reflective finish ideal for high-end jewelry and electronics, whereas steel provides durability and cost-effectiveness for industrial applications. Selecting rhodium ensures superior tarnish protection and enhanced appearance but comes at a higher price point compared to steel's mechanical strength and versatility. The decision depends on balancing budget constraints, desired aesthetic qualities, and the specific environmental exposure of the plated object.
Infographic: Rhodium vs Steel for Electroplating
 azmater.com
azmater.com