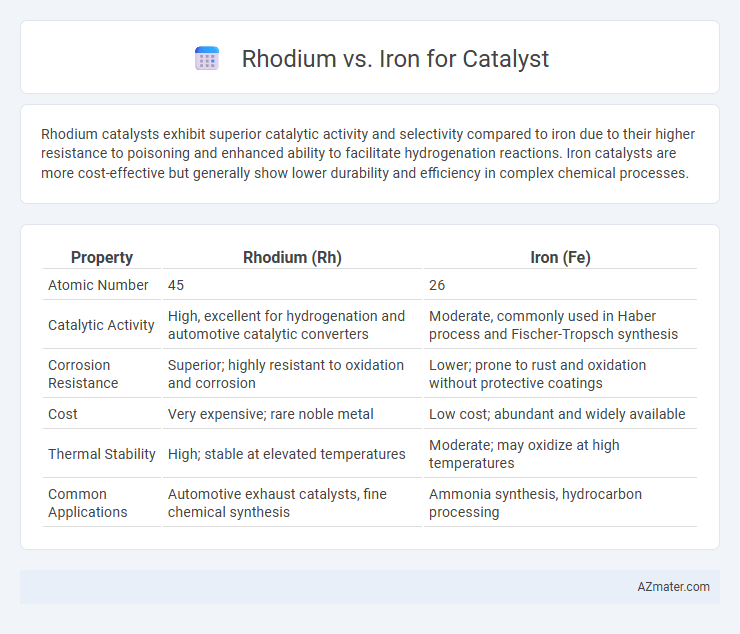
Rhodium catalysts exhibit superior catalytic activity and selectivity compared to iron due to their higher resistance to poisoning and enhanced ability to facilitate hydrogenation reactions. Iron catalysts are more cost-effective but generally show lower durability and efficiency in complex chemical processes.
Table of Comparison
| Property | Rhodium (Rh) | Iron (Fe) |
|---|---|---|
| Atomic Number | 45 | 26 |
| Catalytic Activity | High, excellent for hydrogenation and automotive catalytic converters | Moderate, commonly used in Haber process and Fischer-Tropsch synthesis |
| Corrosion Resistance | Superior; highly resistant to oxidation and corrosion | Lower; prone to rust and oxidation without protective coatings |
| Cost | Very expensive; rare noble metal | Low cost; abundant and widely available |
| Thermal Stability | High; stable at elevated temperatures | Moderate; may oxidize at high temperatures |
| Common Applications | Automotive exhaust catalysts, fine chemical synthesis | Ammonia synthesis, hydrocarbon processing |
Introduction to Rhodium and Iron as Catalysts
Rhodium is a rare, precious metal renowned for its exceptional catalytic activity in industrial processes such as catalytic converters and hydrogenation reactions, offering high selectivity and resistance to poisoning. Iron, an abundant and cost-effective transition metal, serves as a versatile catalyst in applications like Haber-Bosch ammonia synthesis and Fischer-Tropsch reactions, valued for its affordability and scalability despite lower catalytic efficiency. Both metals play critical roles in heterogeneous catalysis, with rhodium excelling in high-performance, sensitive environments, while iron dominates large-scale, cost-sensitive catalytic processes.
Chemical Properties Impacting Catalytic Activity
Rhodium exhibits superior catalytic activity compared to iron due to its higher resistance to oxidation and excellent ability to facilitate hydrogenation reactions, stemming from its d-electron configuration and surface energy characteristics. Iron's catalytic performance often diminishes under oxidative conditions because of its susceptibility to rusting and formation of inactive oxide layers, limiting its effectiveness in prolonged catalytic cycles. The distinct electronic structures of rhodium and iron profoundly influence adsorption energies and reaction intermediates stabilization, making rhodium more efficient in catalytic processes such as hydrogenation and carbon monoxide oxidation.
Common Industrial Applications of Rhodium and Iron Catalysts
Rhodium catalysts are extensively used in catalytic converters for automotive exhaust systems and in the production of nitric acid through the Ostwald process due to their exceptional ability to facilitate oxidation reactions with high selectivity. Iron catalysts are commonly employed in the Haber-Bosch process for ammonia synthesis, playing a critical role in nitrogen fixation at industrial scales, as well as in Fischer-Tropsch synthesis for converting syngas into hydrocarbons. The distinct catalytic properties of rhodium and iron make them indispensable in refining processes, with rhodium favored for hydrogenation and oxidation reactions, while iron excels in large-scale ammonia production and hydrocarbon formation.
Reactivity and Selectivity Comparison
Rhodium catalysts exhibit higher reactivity than iron catalysts, enabling faster reaction rates in processes like hydrogenation and hydroformylation. The superior selectivity of rhodium reduces by-product formation, making it ideal for producing fine chemicals with precision. Iron catalysts, while less selective, offer cost-effective alternatives for large-scale reactions but often require harsher conditions to achieve comparable conversions.
Cost Efficiency and Availability
Rhodium catalysts exhibit superior catalytic activity and selectivity but are significantly more expensive and less abundant than iron catalysts, impacting cost efficiency in large-scale applications. Iron is widely available and far more economical, making it the preferred choice for industrial processes prioritizing budget and resource accessibility despite its lower catalytic performance. The trade-off between rhodium's high cost and scarcity versus iron's affordability and abundance determines their suitability based on specific catalytic requirements and economic constraints.
Environmental and Sustainability Considerations
Rhodium catalysts exhibit superior efficiency and selectivity in reducing harmful emissions during chemical reactions, making them highly effective for automotive catalytic converters with a lower environmental footprint. In contrast, iron catalysts, abundant and less expensive, offer sustainable advantages by minimizing resource depletion and promoting eco-friendly industrial processes despite lower catalytic performance. The choice between rhodium and iron catalysts hinges on balancing the environmental impact of metal extraction and catalyst longevity against emission reduction efficacy.
Stability and Longevity in Catalytic Processes
Rhodium exhibits superior stability and longevity compared to iron in catalytic processes due to its high resistance to oxidation and thermal degradation. Rhodium catalysts maintain activity over extended cycles, enhancing process efficiency in reactions such as hydrogenation and carbonylation. In contrast, iron catalysts often suffer from faster deactivation caused by surface oxidation and sintering, reducing their operational lifespan in industrial applications.
Catalyst Preparation and Handling
Rhodium catalysts require meticulous handling due to their high cost and sensitivity to contamination, often prepared through impregnation or co-precipitation with controlled reduction processes to maintain active sites. Iron catalysts offer greater tolerance to atmospheric exposure and simpler preparation methods such as direct oxidation or precipitation, but necessitate precise control of particle size and phase to optimize catalytic activity. Proper storage and activation are critical for both metals to prevent deactivation and ensure longevity in catalytic applications.
Recent Innovations in Rhodium and Iron Catalysis
Recent innovations in rhodium catalysis have enabled enhanced selectivity and efficiency in complex organic transformations, particularly in C-H activation and asymmetric synthesis. Iron catalysis developments prioritize sustainability, utilizing earth-abundant iron complexes for cost-effective and environmentally benign alternatives to precious metals. Advances in ligand design and reaction engineering continue to expand the scope and applicability of both rhodium and iron catalysts in pharmaceutical and industrial processes.
Choosing the Right Catalyst: Factors to Consider
Rhodium catalysts offer superior activity and selectivity in hydrogenation reactions compared to iron catalysts, making them ideal for fine chemical synthesis despite higher costs. Iron catalysts provide a more economical and environmentally friendly option, with robust performance in Fischer-Tropsch synthesis and ammonia production. Choosing the right catalyst depends on factors such as reaction specificity, cost constraints, catalytic lifespan, and environmental impact.
Infographic: Rhodium vs Iron for Catalyst
 azmater.com
azmater.com