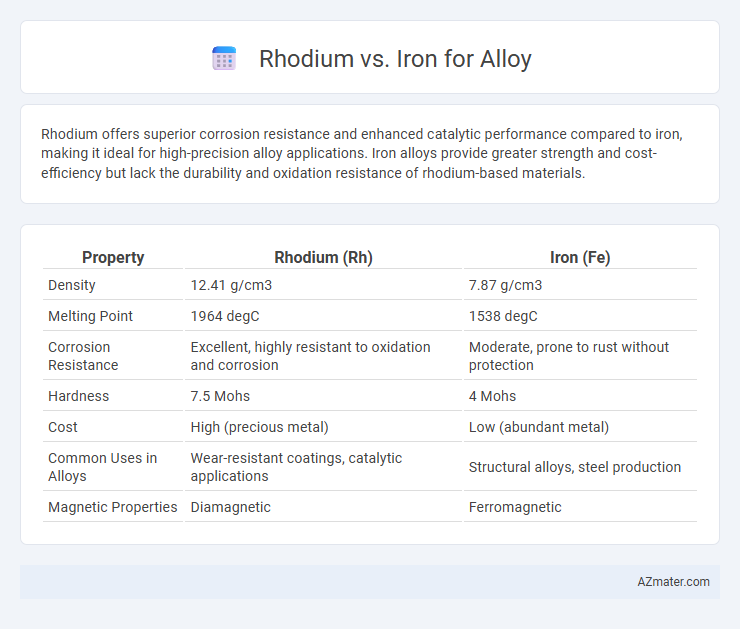
Rhodium offers superior corrosion resistance and enhanced catalytic performance compared to iron, making it ideal for high-precision alloy applications. Iron alloys provide greater strength and cost-efficiency but lack the durability and oxidation resistance of rhodium-based materials.
Table of Comparison
| Property | Rhodium (Rh) | Iron (Fe) |
|---|---|---|
| Density | 12.41 g/cm3 | 7.87 g/cm3 |
| Melting Point | 1964 degC | 1538 degC |
| Corrosion Resistance | Excellent, highly resistant to oxidation and corrosion | Moderate, prone to rust without protection |
| Hardness | 7.5 Mohs | 4 Mohs |
| Cost | High (precious metal) | Low (abundant metal) |
| Common Uses in Alloys | Wear-resistant coatings, catalytic applications | Structural alloys, steel production |
| Magnetic Properties | Diamagnetic | Ferromagnetic |
Overview of Rhodium and Iron in Alloys
Rhodium is a rare, precious metal known for its exceptional corrosion resistance, high melting point, and brilliant reflective properties, making it valuable in catalytic converters and high-performance alloys. Iron, a more abundant transition metal, is prized for its strength, ductility, and magnetic properties, forming the backbone of steel alloys that dominate construction and manufacturing industries. Alloying rhodium with iron can enhance corrosion resistance and thermal stability, although iron's versatility and lower cost maintain its primary role in structural and industrial alloys.
Chemical Properties: Rhodium vs Iron
Rhodium exhibits superior chemical stability and corrosion resistance compared to iron, due to its inertness and resistance to oxidation at high temperatures. Iron, while highly reactive and prone to rust in the presence of moisture and oxygen, forms various oxides affecting alloy durability. The distinct electron configurations of rhodium (4d^8 5s^1) and iron (3d^6 4s^2) influence their differing catalytic properties and chemical reactivity in alloy formation.
Physical Characteristics Comparison
Rhodium exhibits a high melting point of approximately 1964degC and exceptional corrosion resistance, making it significantly more durable than iron, which melts at about 1538degC and is prone to rust. Rhodium's density is around 12.41 g/cm3, considerably higher than iron's 7.87 g/cm3, contributing to its superior hardness and wear resistance in alloys. Unlike iron alloys that often contain carbon to enhance strength, rhodium alloys maintain excellent electrical conductivity and reflectivity, essential for specialized industrial coatings.
Alloy Formation: Compatibility and Processes
Rhodium exhibits excellent compatibility with other platinum-group metals, allowing it to form stable, corrosion-resistant alloys through processes like melting and powder metallurgy. Iron alloys typically require controlled cooling and specific heat treatments to achieve desired mechanical properties and phase stability, with compatibility hinging on carbon content and alloying elements. The formation of rhodium-iron alloys is challenging due to limited mutual solubility and distinct crystallographic structures, necessitating advanced techniques such as high-energy ball milling or sputtering for homogeneous alloy synthesis.
Strength and Durability Factors
Rhodium alloys exhibit superior strength and exceptional corrosion resistance compared to iron-based alloys, making them ideal for high-stress, high-wear applications. Iron, while more abundant and cost-effective, typically requires alloying with carbon or other elements to enhance hardness and durability but remains prone to oxidation and wear over time. Rhodium's inherent hardness contributes to greater longevity in harsh environments, whereas iron alloys offer versatility with moderate strength and durability suited for a wide range of structural uses.
Corrosion Resistance: Rhodium vs Iron
Rhodium exhibits superior corrosion resistance compared to iron, making it highly effective in preventing oxidation and tarnishing in alloy applications. While iron alloys are prone to rust and degradation when exposed to moisture and oxygen, rhodium maintains stability and protects underlying metals from corrosive environments. This characteristic makes rhodium an ideal choice for enhancing durability and longevity in high-performance corrosion-resistant alloys.
Industrial Applications of Rhodium and Iron Alloys
Rhodium alloys excel in high-temperature and corrosion-resistant applications, making them ideal for automotive catalytic converters and electrical contacts where durability is crucial. Iron alloys, particularly steel, dominate structural and manufacturing industries due to their strength, versatility, and cost-effectiveness in construction, machinery, and transportation sectors. Industrial use of rhodium is more specialized and limited compared to the widespread application of iron alloys, which support large-scale infrastructure and heavy industry.
Cost and Availability Considerations
Rhodium is significantly more expensive than iron due to its rarity and complex extraction process, making it less viable for cost-sensitive alloy applications. Iron, abundant and widely available, offers a cost-effective base metal for industrial alloys with reliable supply chains. While rhodium enhances corrosion resistance and catalytic properties, its high price and limited availability often restrict its use to specialized, high-performance alloys.
Environmental Impact and Sustainability
Rhodium, a rare precious metal, has a significantly lower environmental footprint per unit weight compared to iron due to its high recyclability and resistance to corrosion, which reduces resource depletion and waste generation in alloys. Iron, while abundant and easier to extract, requires extensive mining and refining processes that contribute to higher carbon emissions and ecological disturbance. Choosing rhodium-iron alloys can balance sustainability by enhancing durability and reducing frequent replacements, minimizing overall environmental impact.
Choosing the Right Metal: Rhodium or Iron for Alloys
Rhodium offers exceptional corrosion resistance and high reflectivity, making it ideal for specialized alloys used in catalytic converters and jewelry plating, while iron provides excellent tensile strength and cost-effectiveness for structural and industrial alloys. Selecting rhodium alloys enhances durability and aesthetic appeal in high-performance applications, whereas iron alloys are preferred for their versatility and abundance in construction and manufacturing. Consider application-specific requirements such as environmental exposure, mechanical performance, and budget constraints to determine the optimal metal for alloy composition.
Infographic: Rhodium vs Iron for Alloy
 azmater.com
azmater.com