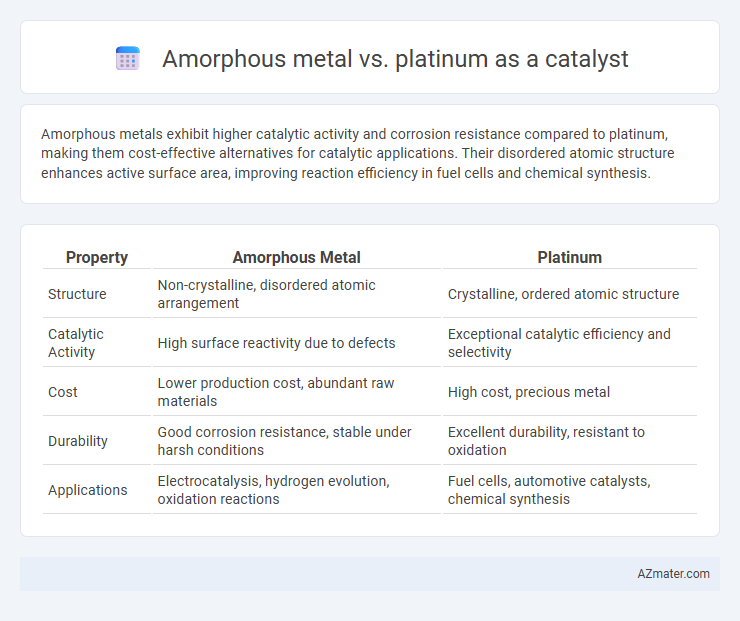
Amorphous metals exhibit higher catalytic activity and corrosion resistance compared to platinum, making them cost-effective alternatives for catalytic applications. Their disordered atomic structure enhances active surface area, improving reaction efficiency in fuel cells and chemical synthesis.
Table of Comparison
| Property | Amorphous Metal | Platinum |
|---|---|---|
| Structure | Non-crystalline, disordered atomic arrangement | Crystalline, ordered atomic structure |
| Catalytic Activity | High surface reactivity due to defects | Exceptional catalytic efficiency and selectivity |
| Cost | Lower production cost, abundant raw materials | High cost, precious metal |
| Durability | Good corrosion resistance, stable under harsh conditions | Excellent durability, resistant to oxidation |
| Applications | Electrocatalysis, hydrogen evolution, oxidation reactions | Fuel cells, automotive catalysts, chemical synthesis |
Introduction to Amorphous Metals and Platinum Catalysts
Amorphous metals, also known as metallic glasses, are non-crystalline materials characterized by a disordered atomic structure that enhances catalytic activity due to increased surface defects and active sites. Platinum catalysts, widely used in industrial applications, exhibit exceptional catalytic performance because of their crystalline structure with well-defined surface facets and high resistance to poisoning. Comparing the two, amorphous metals offer advantages in cost and structural flexibility, while platinum remains the benchmark for catalytic efficiency and stability in reactions such as hydrogen evolution and fuel cell processes.
Structural Differences: Amorphous vs Crystalline Platinum
Amorphous metals exhibit a non-crystalline, disordered atomic structure, unlike crystalline platinum, which has a well-defined, repeating lattice arrangement. This structural difference in amorphous metals leads to a higher density of unsaturated atomic sites and increased surface irregularities, enhancing catalytic activity and resistance to corrosion compared to crystalline platinum. The lack of grain boundaries in amorphous metals also reduces susceptibility to sintering, promoting long-term stability in catalytic applications.
Catalytic Efficiency Comparison
Amorphous metals exhibit higher catalytic efficiency than platinum due to their unique disordered atomic structure, which provides a larger number of active sites and enhanced surface reactivity. Platinum, while traditionally valued for its stable catalytic properties, often suffers from limited active surface area and higher susceptibility to poisoning in reactions such as hydrogen evolution and oxygen reduction. Studies indicate amorphous metal catalysts can achieve increased turnover frequencies and improved resistance to deactivation, making them a promising alternative in catalytic applications.
Durability and Stability in Catalytic Applications
Amorphous metals exhibit superior durability and stability in catalytic applications compared to platinum due to their unique non-crystalline atomic structure, which enhances resistance to corrosion and sintering under harsh reaction conditions. Platinum catalysts often suffer from particle agglomeration and surface deactivation, reducing their long-term effectiveness. The enhanced structural stability of amorphous metals leads to prolonged catalytic performance and lower degradation rates, making them promising alternatives for durable catalyst systems.
Cost Analysis: Amorphous Metals vs Platinum
Amorphous metals offer a significant cost advantage over platinum in catalyst applications due to their lower raw material expenses and scalable manufacturing processes. Platinum catalysts, while highly effective, are substantially more expensive because of limited supply and complex extraction methods. The economic feasibility of amorphous metals makes them an attractive alternative for large-scale industrial catalysis, reducing overall production costs without compromising performance.
Environmental Impact and Sustainability
Amorphous metals exhibit superior catalytic efficiency with reduced environmental toxicity due to their lower reliance on scarce and expensive metals like platinum. Platinum catalysts, while highly effective, pose sustainability challenges because of limited natural reserves and environmentally harmful mining processes. Transitioning to amorphous metal catalysts can significantly lower ecological footprints and support sustainable industrial applications by minimizing resource depletion and hazardous waste.
Applications in Industry and Research
Amorphous metals exhibit superior catalytic efficiency in hydrogenation and oxidation reactions due to their high surface area and unique atomic structure, making them ideal for industrial chemical synthesis and energy conversion processes. Platinum remains a benchmark catalyst in fuel cells and automotive exhaust treatment because of its exceptional stability and activity under harsh conditions, despite higher costs. Research continues to explore amorphous metals for scalable, cost-effective alternatives to platinum, aiming to enhance catalytic performance in sustainable energy and environmental applications.
Challenges in Manufacturing and Scalability
Manufacturing amorphous metal catalysts involves challenges such as maintaining their non-crystalline structure during mass production, which requires precise control over cooling rates and alloy composition. Scalability is limited by difficulties in producing uniform materials at large volumes without compromising catalytic performance, whereas platinum catalysts benefit from established synthesis routes but face cost and resource scarcity issues. The trade-off between high catalytic efficiency in amorphous metals and the economic feasibility of platinum pushes ongoing research to optimize fabrication techniques and improve large-scale viability.
Recent Advances in Catalyst Development
Recent advances in catalyst development highlight amorphous metals as promising alternatives to platinum due to their unique disordered atomic structure that enhances catalytic activity and durability, particularly in oxygen reduction reactions (ORR). Unlike crystalline platinum catalysts, amorphous metals exhibit higher resistance to corrosion and poisoning, resulting in improved stability for fuel cells and hydrogen production applications. Innovations in synthesis techniques such as rapid quenching and electrodeposition have enabled precise control over amorphous metal compositions, optimizing their surface area and electronic properties for superior catalytic performance.
Future Prospects and Innovations
Amorphous metal catalysts exhibit superior surface area and tunable electronic properties compared to traditional platinum, driving advancements in energy conversion and storage technologies. Innovations in nanoscale engineering and alloy compositions enhance their catalytic efficiency and durability, potentially reducing reliance on scarce and expensive platinum resources. Future prospects include scalable production methods and integration into fuel cells and electrochemical reactors, promoting sustainable and cost-effective clean energy solutions.
Infographic: Amorphous metal vs Platinum for Catalyst
 azmater.com
azmater.com