Rhodium offers superior corrosion resistance and a brilliant, reflective finish ideal for high-end electroplating applications, while gallium provides a low melting point and excellent wettability suited for niche, low-temperature plating processes. Choosing rhodium enhances durability and luster in jewelry and automotive parts, whereas gallium is preferred for semiconductor and flexible electronics plating.
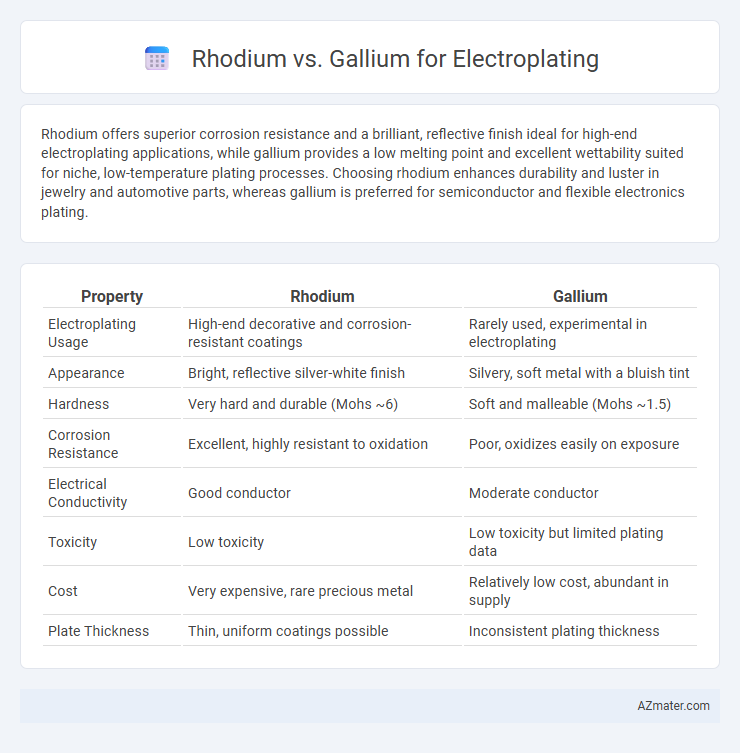
Table of Comparison
| Property | Rhodium | Gallium |
|---|---|---|
| Electroplating Usage | High-end decorative and corrosion-resistant coatings | Rarely used, experimental in electroplating |
| Appearance | Bright, reflective silver-white finish | Silvery, soft metal with a bluish tint |
| Hardness | Very hard and durable (Mohs ~6) | Soft and malleable (Mohs ~1.5) |
| Corrosion Resistance | Excellent, highly resistant to oxidation | Poor, oxidizes easily on exposure |
| Electrical Conductivity | Good conductor | Moderate conductor |
| Toxicity | Low toxicity | Low toxicity but limited plating data |
| Cost | Very expensive, rare precious metal | Relatively low cost, abundant in supply |
| Plate Thickness | Thin, uniform coatings possible | Inconsistent plating thickness |
Introduction to Electroplating: Rhodium vs Gallium
Electroplating uses electrical currents to deposit a metal coating onto a substrate, enhancing corrosion resistance, conductivity, and aesthetics. Rhodium, a rare and highly reflective metal from the platinum group, provides superior hardness and corrosion resistance, making it ideal for jewelry and automotive trims. Gallium, a post-transition metal with low melting point and unique wetting properties, is less common in electroplating but offers promising applications in semiconductors and flexible electronics.
Chemical Properties: Rhodium and Gallium Overview
Rhodium, a rare platinum-group metal, exhibits exceptional chemical stability, corrosion resistance, and a high melting point of 1964degC, making it ideal for durable, reflective electroplating. Gallium, a post-transition metal with a melting point of just 29.76degC, offers unique low-temperature plating capabilities and excellent adherence to substrates but is less chemically inert than rhodium. The strong oxidation resistance of rhodium contrasts with gallium's tendency to oxidize and form a protective oxide layer, influencing their suitability for various electroplating applications based on environmental and performance requirements.
Electroplating Processes for Rhodium and Gallium
Rhodium electroplating involves the deposition of a thin, highly reflective, and corrosion-resistant rhodium layer using a cyanide-based electrolyte bath typically maintained at 30-50degC with controlled current density between 1-3 A/dm2. Gallium electroplating, less common and more challenging due to gallium's low melting point and reactive nature, uses non-aqueous electrolytes such as ionic liquids or organic solvents to achieve uniform coatings, often requiring precise temperature control below 50degC to prevent substrate damage. The rhodium process is valued for jewelry and automotive applications for its hardness and tarnish resistance, while gallium electroplating is experimental, focusing on semiconductor and flexible electronics with an emphasis on producing thin, conductive, and corrosion-resistant films.
Performance Comparison: Rhodium vs Gallium Coatings
Rhodium coatings exhibit superior hardness, corrosion resistance, and reflectivity, making them ideal for high-wear applications and jewelry electroplating. Gallium coatings provide excellent conductivity and improved adhesion on specific substrates but generally lack the durability and oxidation resistance of rhodium. The choice between rhodium and gallium depends on balancing durability and electrical performance based on application requirements.
Corrosion Resistance: Rhodium vs Gallium
Rhodium offers superior corrosion resistance compared to gallium, making it the preferred choice for electroplating applications requiring durable, long-lasting finishes. While rhodium forms a hard, inert surface that resists oxidation and tarnishing, gallium's softer nature and higher reactivity limit its effectiveness against corrosion. Consequently, rhodium plating is widely used in jewelry and electronics for enhanced protection and extended lifespan.
Cost and Availability Analysis
Rhodium offers superior corrosion resistance and a brilliant finish for electroplating but comes at a significantly higher cost due to its rarity and extensive mining process. Gallium presents a more affordable and abundant alternative, though its plating durability and conductivity are comparatively lower, which can limit its industrial applications. The choice between rhodium and gallium plating ultimately hinges on balancing budget constraints with the required performance and longevity of the finish.
Environmental Impact of Rhodium and Gallium Electroplating
Rhodium electroplating involves the use of toxic chemicals like rhodium chloride and requires careful waste management due to the heavy metal's high toxicity and environmental persistence. Gallium electroplating presents lower environmental risks as gallium is less toxic and more abundant, reducing ecological hazards related to mining and disposal. Both processes necessitate strict adherence to environmental regulations, but gallium offers a more sustainable alternative with reduced impact on water and soil contamination.
Industrial Applications: Rhodium vs Gallium
Rhodium is extensively used in industrial electroplating due to its exceptional hardness, corrosion resistance, and brilliant reflective finish, making it ideal for automotive, jewelry, and electronics industries. Gallium, while less common for electroplating, offers potential in specialized applications requiring low melting point alloys and semiconductor properties but lacks the durability and conductivity of rhodium coatings. Industrial applications favor rhodium for robust, wear-resistant surfaces, whereas gallium's role remains more experimental and niche within electroplating technologies.
Safety Considerations in Electroplating
Rhodium offers superior corrosion resistance and minimal toxicity, making it a safer choice for electroplating compared to gallium, which poses risks due to its toxicity and potential environmental hazards. Proper ventilation and protective equipment are essential when handling gallium electroplating solutions, as exposure can cause skin irritation and respiratory issues. Rhodium electroplating processes require less stringent safety protocols, reducing occupational health risks in industrial applications.
Choosing the Right Metal: Rhodium or Gallium for Electroplating
Rhodium offers superior corrosion resistance, high reflectivity, and exceptional hardness, making it ideal for jewelry and automotive finishes requiring durability and a bright, mirror-like appearance. Gallium, with its low melting point and unique wetting properties, is primarily used in specialized electronic and semiconductor applications where precise thin-film deposition is essential. Selecting between rhodium and gallium depends on the specific electroplating requirements, such as wear resistance, conductivity, and thermal stability.
Infographic: Rhodium vs Gallium for Electroplating
 azmater.com
azmater.com