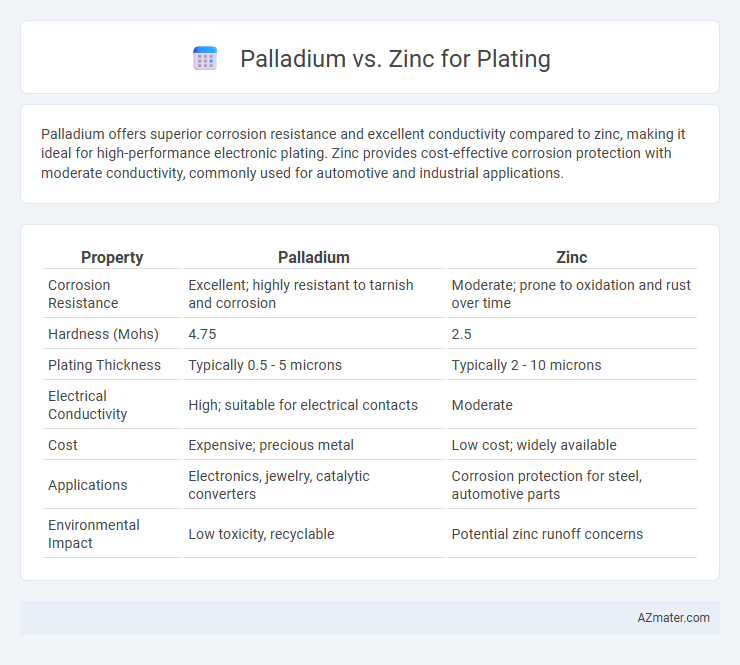
Palladium offers superior corrosion resistance and excellent conductivity compared to zinc, making it ideal for high-performance electronic plating. Zinc provides cost-effective corrosion protection with moderate conductivity, commonly used for automotive and industrial applications.
Table of Comparison
| Property | Palladium | Zinc |
|---|---|---|
| Corrosion Resistance | Excellent; highly resistant to tarnish and corrosion | Moderate; prone to oxidation and rust over time |
| Hardness (Mohs) | 4.75 | 2.5 |
| Plating Thickness | Typically 0.5 - 5 microns | Typically 2 - 10 microns |
| Electrical Conductivity | High; suitable for electrical contacts | Moderate |
| Cost | Expensive; precious metal | Low cost; widely available |
| Applications | Electronics, jewelry, catalytic converters | Corrosion protection for steel, automotive parts |
| Environmental Impact | Low toxicity, recyclable | Potential zinc runoff concerns |
Introduction to Metal Plating: Palladium vs Zinc
Palladium and zinc are widely used metals in plating applications due to their unique properties and benefits. Palladium plating offers excellent corrosion resistance, high durability, and a brilliant silver-white finish, making it ideal for electronic connectors and jewelry. Zinc plating provides cost-effective protection against rust and enhances adhesion for subsequent coatings, commonly used in automotive and construction industries.
Chemical Properties of Palladium and Zinc
Palladium exhibits excellent corrosion resistance due to its noble metal characteristics, maintaining stability in both acidic and basic environments, while its atomic number 46 and electron configuration [Kr] 4d10 enable effective catalytic properties. Zinc, with atomic number 30 and electron configuration [Ar] 3d10 4s2, offers good corrosion resistance through the formation of a protective oxide layer but is more reactive, making it prone to oxidation under certain conditions. The chemical inertness of palladium makes it ideal for high-performance plating applications requiring durability and resistance to tarnish, whereas zinc plating is favored for sacrificial protection due to its anodic behavior relative to iron.
Plating Process: Palladium vs Zinc Techniques
Palladium plating involves an electrochemical deposition process that creates a thin, corrosion-resistant layer, often applied via an electroless or electroplating method for enhanced conductivity and durability. Zinc plating typically uses electroplating or hot-dip methods to produce a sacrificial, protective coating that prevents rust and extends the lifespan of steel parts. Both techniques require surface preparation such as cleaning and activation, but palladium plating offers superior oxidation resistance, while zinc is favored for cost-effective, large-scale corrosion protection.
Corrosion Resistance Comparison
Palladium plating offers superior corrosion resistance compared to zinc plating due to its inert nature and excellent resistance to oxidation and tarnishing, making it ideal for harsh environments and electronic components. Zinc plating provides good corrosion protection primarily through sacrificial galvanic action, which prevents rust but tends to degrade faster under prolonged exposure to moisture and chemicals. The higher corrosion resistance of palladium contributes to longer-lasting protection and enhanced durability in critical applications.
Electrical Conductivity in Plating Applications
Palladium plating offers superior electrical conductivity compared to zinc, making it ideal for high-performance electronic components and connectors. Zinc plating provides corrosion resistance but has lower conductivity, limiting its use in applications where efficient electrical flow is critical. The choice between palladium and zinc depends on balancing cost with the required conductivity levels in plating applications.
Environmental Impact and Sustainability
Palladium plating offers exceptional corrosion resistance and conductivity but involves energy-intensive mining and limited recyclability, raising environmental concerns. Zinc plating provides a more sustainable alternative due to abundant availability, lower energy consumption during extraction, and better recyclability, minimizing ecological footprint. Choosing zinc over palladium for plating applications supports greener manufacturing practices and reduces hazardous waste generation.
Cost Analysis: Palladium vs Zinc Plating
Palladium plating is significantly more expensive than zinc plating due to the higher raw material cost and processing complexity, with palladium often costing several times more per ounce compared to zinc. Zinc plating offers a cost-effective solution for corrosion resistance in mass production, making it ideal for automotive and construction applications where budget constraints are critical. While palladium provides superior wear resistance and aesthetic appeal for high-end electronics and jewelry, its cost premium limits its use to specialized applications where performance justifies the expense.
Common Industrial Uses and Applications
Palladium plating is widely used in electronics manufacturing for enhancing corrosion resistance and conductivity in connectors, contacts, and circuit boards, especially in high-reliability industries like aerospace and automotive. Zinc plating is predominantly employed for corrosion protection of steel components in construction, automotive, and hardware industries due to its sacrificial anode properties. Both metals serve crucial roles in industrial coatings, with palladium favored for precision electronic applications and zinc chosen for cost-effective, large-scale rust prevention.
Durability and Wear Resistance
Palladium plating offers superior durability and excellent wear resistance, making it ideal for high-use applications where corrosion protection and surface hardness are critical. Zinc plating provides moderate durability with good wear resistance primarily in less demanding environments, offering cost-effective corrosion protection. The choice between palladium and zinc plating depends on the required lifespan and exposure conditions, with palladium excelling in high-performance, long-lasting finishes.
Choosing the Right Plating Material for Your Project
Choosing between palladium and zinc for plating depends on factors like corrosion resistance, cost, and application environment. Palladium offers superior corrosion resistance and excellent conductivity, making it ideal for high-performance electronics and jewelry, while zinc provides cost-effective protection primarily against rust in automotive and industrial components. Evaluate the specific requirements of your project, including durability needs and budget constraints, to select the most suitable plating material.
Infographic: Palladium vs Zinc for Plating
 azmater.com
azmater.com