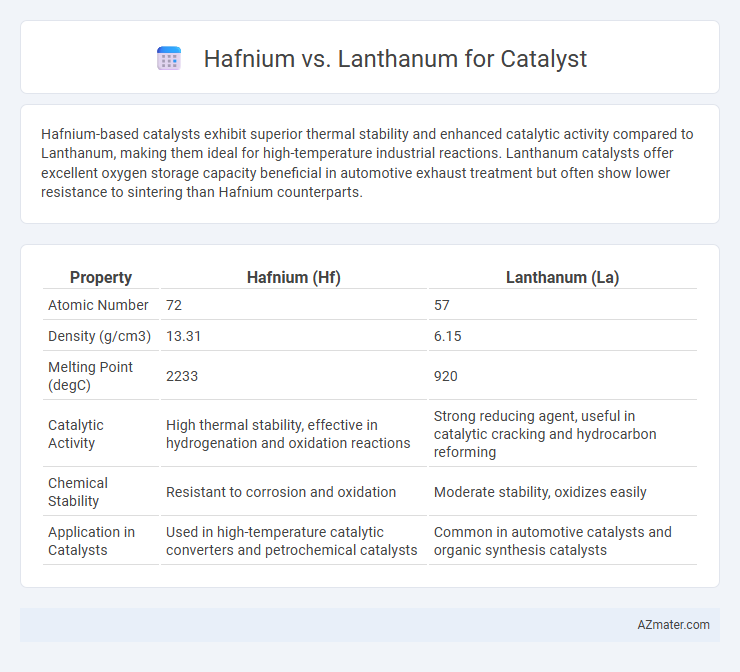
Hafnium-based catalysts exhibit superior thermal stability and enhanced catalytic activity compared to Lanthanum, making them ideal for high-temperature industrial reactions. Lanthanum catalysts offer excellent oxygen storage capacity beneficial in automotive exhaust treatment but often show lower resistance to sintering than Hafnium counterparts.
Table of Comparison
| Property | Hafnium (Hf) | Lanthanum (La) |
|---|---|---|
| Atomic Number | 72 | 57 |
| Density (g/cm3) | 13.31 | 6.15 |
| Melting Point (degC) | 2233 | 920 |
| Catalytic Activity | High thermal stability, effective in hydrogenation and oxidation reactions | Strong reducing agent, useful in catalytic cracking and hydrocarbon reforming |
| Chemical Stability | Resistant to corrosion and oxidation | Moderate stability, oxidizes easily |
| Application in Catalysts | Used in high-temperature catalytic converters and petrochemical catalysts | Common in automotive catalysts and organic synthesis catalysts |
Introduction to Hafnium and Lanthanum Catalysts
Hafnium catalysts exhibit remarkable thermal stability and strong Lewis acidity, making them highly effective in polymerization and organic synthesis reactions. Lanthanum catalysts, recognized for their unique electronic configuration and large ionic radius, excel in redox reactions and exhibit excellent catalytic activity in environmental and energy applications. Both metals offer distinct catalytic properties, with hafnium favoring acid-catalyzed processes, while lanthanum is suited for oxidation and reduction reactions.
Elemental Properties Relevant to Catalysis
Hafnium exhibits a high melting point (about 2233degC) and strong resistance to corrosion, which enhances its durability as a catalyst under extreme reaction conditions. Its electronic configuration [Xe] 4f14 5d2 6s2 facilitates effective d-orbital participation in catalytic cycles, promoting strong metal-ligand interactions essential for catalytic activity. Lanthanum, with a lower melting point (about 920degC) and an electronic configuration of [Xe] 5d1 6s2, typically shows higher ionic radius and weaker metal-ligand bonding, which may limit its catalytic effectiveness in high-temperature or electron-intensive reactions.
Common Applications in Catalytic Processes
Hafnium is widely used in catalytic converters and hydrogenation reactions due to its high thermal stability and strong Lewis acidity, making it effective in olefin polymerization and hydrocarbon reforming. Lanthanum plays a critical role in automotive catalytic converters and oxidative coupling of methane, valued for its oxygen storage capacity and ability to enhance catalyst durability. Both elements are vital in refining processes, with hafnium preferred for high-temperature applications and lanthanum enhancing catalyst performance in oxidation and reduction reactions.
Catalytic Efficiency: Hafnium vs Lanthanum
Hafnium-based catalysts demonstrate superior catalytic efficiency compared to lanthanum in various chemical reactions due to their higher electron density and stronger Lewis acidity, which enhance substrate activation. Lanthanum catalysts, while effective in promoting basic reactions, often exhibit lower turnover frequencies and reduced stability under harsh conditions relative to hafnium counterparts. The distinct electronic configurations and coordination environments of hafnium enable more efficient catalytic cycles, especially in olefin polymerization and selective oxidation processes.
Stability and Longevity in Reaction Conditions
Hafnium-based catalysts exhibit superior thermal and chemical stability compared to lanthanum catalysts under harsh reaction conditions, enabling prolonged operational lifespans. The d-electron configuration of hafnium enhances resistance to sintering and deactivation, maintaining catalytic activity over extended cycles. Lanthanum catalysts, while effective, tend to degrade faster in oxidative or high-temperature environments, limiting their longevity in industrial applications.
Selectivity and Reaction Specificity
Hafnium-based catalysts exhibit superior selectivity and reaction specificity compared to lanthanum counterparts due to their stronger Lewis acidity and ability to stabilize transition states in various organic transformations. The unique electronic configuration of hafnium enhances its efficacy in reactions requiring precise control, such as alkene metathesis and polymerization, resulting in higher yield and reduced by-products. Lanthanum catalysts, while effective in broader applications, often show lower specificity, leading to less controlled product distributions in complex catalytic processes.
Cost and Commercial Availability
Hafnium catalysts, though highly effective in specific high-temperature applications, tend to be significantly more expensive and less commercially available compared to lanthanum-based catalysts. Lanthanum offers a more cost-efficient option with broader industrial supply due to its abundance and established production processes. The economic advantage and accessibility of lanthanum make it the preferred choice for large-scale catalytic applications.
Environmental Impact and Sustainability
Hafnium-based catalysts exhibit superior environmental benefits due to their higher stability and recyclability, reducing waste generation compared to lanthanum catalysts that often require more frequent replacement. Hafnium's lower toxicity and resistance to leaching minimize ecological contamination during catalytic processes, enhancing sustainability in industrial applications. Lanthanum catalysts, while effective, pose challenges in disposal and resource depletion, making hafnium a more sustainable choice for eco-friendly catalytic systems.
Recent Advances and Research Trends
Hafnium-based catalysts exhibit superior thermal stability and unique electronic properties that enhance catalytic activity in hydrocarbon reforming and polymerization reactions, as shown in recent studies published in journals like *Catalysis Science & Technology*. Lanthanum catalysts, with their strong oxygen storage capacity and adjustable oxidation states, have gained attention for applications in automotive exhaust treatment and selective hydrogenation processes, benefiting from advances in nanostructuring techniques. Current research trends emphasize the development of hafnium-lanthanum composite catalysts to synergistically improve performance in environmental catalysis and energy conversion systems.
Future Outlook: Industrial and Scientific Potential
Hafnium exhibits promising catalytic properties due to its high thermal stability and resistance to corrosion, making it ideal for harsh industrial environments such as petrochemical refining and hydrogen production. Lanthanum offers significant advantages in catalysis for sustainable energy applications, particularly in solid oxide fuel cells and automotive catalytic converters, owing to its oxygen storage capacity and redox behavior. Future developments in nanostructured catalysts and alloy compositions are expected to enhance the efficiency and selectivity of both hafnium- and lanthanum-based catalysts, driving innovation in green technologies and advanced material synthesis.
Infographic: Hafnium vs Lanthanum for Catalyst
 azmater.com
azmater.com