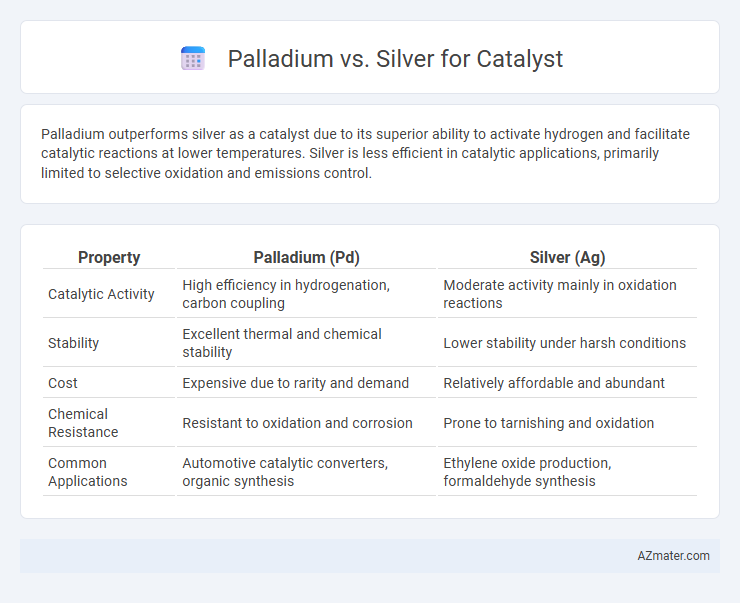
Palladium outperforms silver as a catalyst due to its superior ability to activate hydrogen and facilitate catalytic reactions at lower temperatures. Silver is less efficient in catalytic applications, primarily limited to selective oxidation and emissions control.
Table of Comparison
| Property | Palladium (Pd) | Silver (Ag) |
|---|---|---|
| Catalytic Activity | High efficiency in hydrogenation, carbon coupling | Moderate activity mainly in oxidation reactions |
| Stability | Excellent thermal and chemical stability | Lower stability under harsh conditions |
| Cost | Expensive due to rarity and demand | Relatively affordable and abundant |
| Chemical Resistance | Resistant to oxidation and corrosion | Prone to tarnishing and oxidation |
| Common Applications | Automotive catalytic converters, organic synthesis | Ethylene oxide production, formaldehyde synthesis |
Introduction to Catalytic Materials: Palladium vs Silver
Palladium exhibits superior catalytic properties compared to silver due to its higher activity in hydrogenation and oxidation reactions, making it a preferred choice in automotive catalytic converters and chemical synthesis. Silver, while less active, offers cost advantages and specific selectivity in processes such as ethylene epoxidation and formaldehyde production. The distinct electronic structures and surface adsorption characteristics of palladium and silver directly influence their catalytic efficiency and applications in industrial chemical reactions.
Chemical Properties and Reactivity of Palladium and Silver
Palladium exhibits superior catalytic properties compared to silver due to its higher chemical reactivity and ability to adsorb hydrogen efficiently, facilitating catalytic hydrogenation reactions. While silver has moderate catalytic activity primarily due to its lower affinity for hydrogen and limited ability to activate molecular oxygen, palladium forms surface hydrides and exhibits variable oxidation states (Pd(0), Pd(II)), enhancing its versatility in catalysis. The electronic configuration and d-band structure of palladium enable rapid electron transfer and stronger substrate coordination, making it a preferred catalyst in cross-coupling and carbon-carbon bond-forming reactions, whereas silver is mainly limited to oxidation processes.
Catalytic Efficiency in Industrial Applications
Palladium exhibits superior catalytic efficiency compared to silver in industrial applications due to its ability to facilitate hydrogenation and oxidation reactions at lower temperatures with higher selectivity. Silver catalysts are primarily effective in ethylene epoxidation but generally require higher temperatures and offer lower activity rates. The higher cost of palladium is offset by its increased turnover frequency and durability, making it the preferred choice for processes such as automotive catalytic converters and fine chemical synthesis.
Selectivity and Activity in Common Reactions
Palladium exhibits higher catalytic activity and selectivity than silver in hydrogenation and cross-coupling reactions due to its superior ability to activate hydrogen and form organometallic intermediates. Silver catalysts demonstrate moderate activity but are particularly selective in oxidation reactions, such as ethylene epoxidation, benefiting from their distinct electronic properties and surface oxygen species. Optimizing catalyst supports and reaction conditions further enhances palladium's performance in carbon-carbon bond formation while silver excels in partial oxidation processes.
Cost-Effectiveness and Availability Comparison
Palladium catalysts typically exhibit higher activity and selectivity in chemical reactions but come at a significantly higher cost due to their rarity and complex extraction processes. Silver catalysts, though generally less active, offer a more cost-effective alternative with greater availability and lower market volatility, making them suitable for large-scale industrial applications with budget constraints. The choice between palladium and silver depends on balancing performance requirements against budget and supply chain stability.
Stability and Lifespan of Palladium vs Silver Catalysts
Palladium catalysts exhibit superior stability and longer lifespan compared to silver catalysts, as palladium resists oxidation and sintering under high temperatures and harsh reaction conditions. Silver catalysts often suffer from faster deactivation due to surface oxidation and particle agglomeration, leading to reduced catalytic performance over time. The enhanced durability of palladium enables sustained catalytic activity in processes like hydrogenation and carbon-carbon coupling, making it a preferred choice for industrial applications requiring longevity.
Environmental Impact and Sustainability
Palladium catalysts offer high efficiency and selectivity but involve complex extraction processes with significant environmental footprints, including habitat disruption and high energy use. Silver catalysts, while generally less active than palladium, are more abundant and easier to recycle, resulting in lower environmental impact and greater sustainability in large-scale applications. Sustainable catalyst development increasingly favors silver due to its reduced ecological burden and the potential for green synthesis routes.
Common Industries Using Palladium and Silver Catalysts
Palladium catalysts are extensively used in automotive industries for catalytic converters, facilitating the reduction of harmful emissions like carbon monoxide and nitrogen oxides due to their excellent hydrogenation and oxidation properties. Silver catalysts find prominent applications in chemical manufacturing, particularly in ethylene oxide production and formaldehyde synthesis, leveraging their superior oxidation capabilities. Both metals play crucial roles in environmental and industrial processes, with palladium favored in fuel cell technology and silver utilized in selective oxidation reactions.
Advances in Catalyst Design: Palladium vs Silver
Palladium catalysts exhibit superior activity and selectivity in hydrogenation and carbon-carbon coupling reactions due to their unique electronic structure and ability to activate molecular hydrogen efficiently. Silver catalysts demonstrate high performance in oxidation reactions, such as ethylene epoxidation, attributed to their optimal oxygen adsorption properties and lower cost compared to palladium. Recent advances in catalyst design emphasize nanostructuring and alloying palladium with silver to enhance catalytic efficiency, stability, and cost-effectiveness in industrial applications.
Future Trends and Research Directions in Catalysis
Palladium exhibits superior catalytic efficiency and selectivity compared to silver, driving ongoing research toward optimizing palladium-based catalysts for sustainable chemical processes and energy applications. Emerging trends emphasize the development of palladium alloys, nanostructured catalysts, and heterogeneous catalytic systems to enhance activity and recyclability while reducing costs. Future research explores integrating palladium catalysts with green chemistry principles, such as biomass conversion and CO2 reduction, to address environmental challenges and advance renewable energy technologies.
Infographic: Palladium vs Silver for Catalyst
 azmater.com
azmater.com