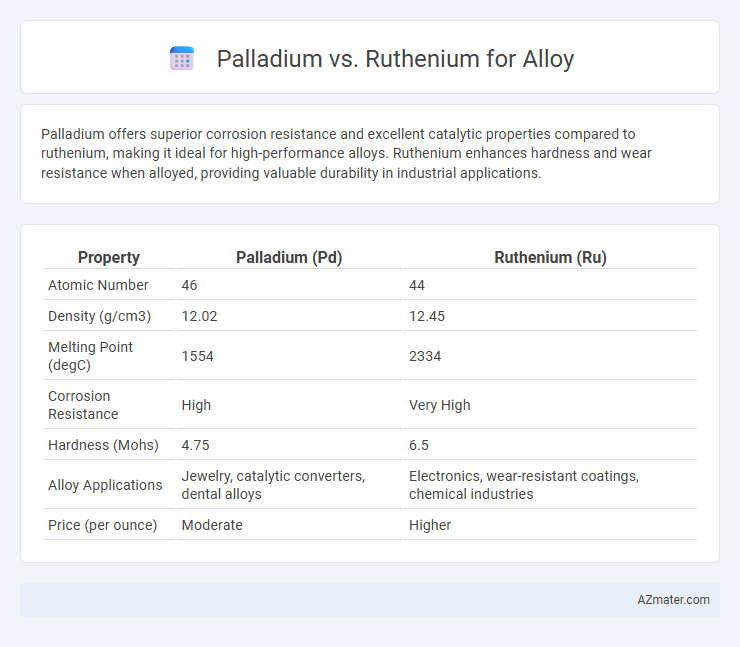
Palladium offers superior corrosion resistance and excellent catalytic properties compared to ruthenium, making it ideal for high-performance alloys. Ruthenium enhances hardness and wear resistance when alloyed, providing valuable durability in industrial applications.
Table of Comparison
| Property | Palladium (Pd) | Ruthenium (Ru) |
|---|---|---|
| Atomic Number | 46 | 44 |
| Density (g/cm3) | 12.02 | 12.45 |
| Melting Point (degC) | 1554 | 2334 |
| Corrosion Resistance | High | Very High |
| Hardness (Mohs) | 4.75 | 6.5 |
| Alloy Applications | Jewelry, catalytic converters, dental alloys | Electronics, wear-resistant coatings, chemical industries |
| Price (per ounce) | Moderate | Higher |
Introduction to Palladium and Ruthenium Alloys
Palladium alloys are prized for their excellent corrosion resistance, high melting point of 1555degC, and exceptional catalytic properties, making them ideal for use in electronics, jewelry, and automotive catalytic converters. Ruthenium alloys offer superior hardness, wear resistance, and stability at temperatures above 2200degC, often used as a hardening agent in platinum and palladium-based alloys for improved durability. Both metals belong to the platinum group and are critical in high-performance applications where strength, corrosion resistance, and catalytic efficiency are essential.
Material Properties Overview
Palladium exhibits excellent corrosion resistance, high ductility, and superior catalytic properties, making it ideal for durable and adaptable alloy compositions. Ruthenium offers exceptional hardness, high melting point, and remarkable resistance to wear and oxidation, enhancing the strength and longevity of metal alloys. Both metals improve alloy performance but are chosen based on specific needs such as flexibility with palladium or increased hardness and thermal stability with ruthenium.
Mechanical Strength Comparison
Palladium alloys exhibit moderate mechanical strength with excellent corrosion resistance, making them suitable for applications requiring durability and resistance to wear. Ruthenium alloys demonstrate significantly higher mechanical strength and hardness, enhancing wear resistance and structural integrity under high-stress conditions. The superior strength of ruthenium-containing alloys makes them ideal for high-performance applications where mechanical robustness is critical.
Corrosion Resistance Analysis
Palladium alloys exhibit superior corrosion resistance in acidic and chloride-rich environments compared to ruthenium alloys, making them ideal for applications requiring long-term durability. Ruthenium, while offering excellent hardness and wear resistance, tends to form less stable passive oxide layers, resulting in lower resistance to oxidation and chemical attack. Alloy compositions with higher palladium content consistently demonstrate enhanced electrochemical stability and reduced susceptibility to pitting corrosion in marine and industrial atmospheres.
Electrical and Thermal Conductivity
Palladium exhibits excellent electrical conductivity of approximately 9.5 x 10^6 S/m, making it highly efficient for alloy applications requiring superior electron flow, while ruthenium offers a lower electrical conductivity around 2.1 x 10^6 S/m but provides exceptional thermal stability and corrosion resistance. In terms of thermal conductivity, ruthenium outperforms palladium with values near 117 W/m*K compared to palladium's 71.8 W/m*K, facilitating better heat dissipation in high-temperature environments. Alloys incorporating palladium are thus preferred for electrical contacts and connectors, whereas ruthenium-based alloys are optimized for applications demanding thermal endurance and minimal electrical resistance under extreme conditions.
Cost and Availability of Palladium vs. Ruthenium
Palladium is generally more expensive than ruthenium due to its higher demand in catalytic converters and electronics, with prices fluctuating around $2,000 to $2,500 per ounce, whereas ruthenium typically costs less, ranging from $300 to $800 per ounce. Ruthenium's greater abundance in the Earth's crust, primarily sourced from platinum group metal deposits in Russia and South Africa, contributes to its lower cost and increased availability compared to palladium, which faces supply constraints from limited major mining regions. The cost-effectiveness and accessibility of ruthenium make it a viable alternative in alloy production where palladium's premium pricing is a limiting factor.
Applications in Industry and Technology
Palladium alloys are extensively used in automotive catalytic converters, electronics, and dental materials due to their excellent corrosion resistance and hydrogen absorption properties. Ruthenium alloys enhance mechanical strength and wear resistance, making them ideal for electrical contacts, hard disk drives, and aerospace components. Both metals significantly improve alloy performance in specialized industrial applications, with palladium excelling in chemical catalysis and ruthenium in durability under extreme conditions.
Environmental Impact and Sustainability
Palladium alloys exhibit lower environmental impact due to their high recyclability and lower toxicity compared to ruthenium alloys, which may involve more energy-intensive extraction processes. Ruthenium, while rarer and harder, contributes to sustainability through its durability in catalytic applications, reducing the need for frequent replacement. Lifecycle assessment reveals palladium's advantages in supply chain efficiency, whereas ruthenium's scarcity raises concerns about sustainable sourcing.
Alloying Behavior and Compatibility
Palladium exhibits excellent alloying behavior with metals such as silver, copper, and gold, enhancing corrosion resistance and ductility, making it highly compatible for use in jewelry and catalytic converters. Ruthenium forms stable alloys primarily with platinum and palladium, improving hardness, wear resistance, and oxidation durability, which is valuable in electrical contacts and high-temperature applications. Both metals demonstrate strong miscibility with platinum-group metals but differ in their affinity for base metals, influencing their selection based on desired mechanical and chemical properties in alloy design.
Choosing the Right Alloy: Factors to Consider
Selecting between palladium and ruthenium alloys involves evaluating factors such as corrosion resistance, hardness, and cost-effectiveness for specific applications. Palladium alloys offer superior corrosion resistance and excellent ductility, making them ideal for jewelry and dental uses, while ruthenium alloys provide enhanced hardness and wear resistance suited for electrical contacts and catalytic converters. Consideration of application requirements, environmental exposure, and budget constraints ensures the optimal alloy choice for durability and performance.
Infographic: Palladium vs Ruthenium for Alloy
 azmater.com
azmater.com