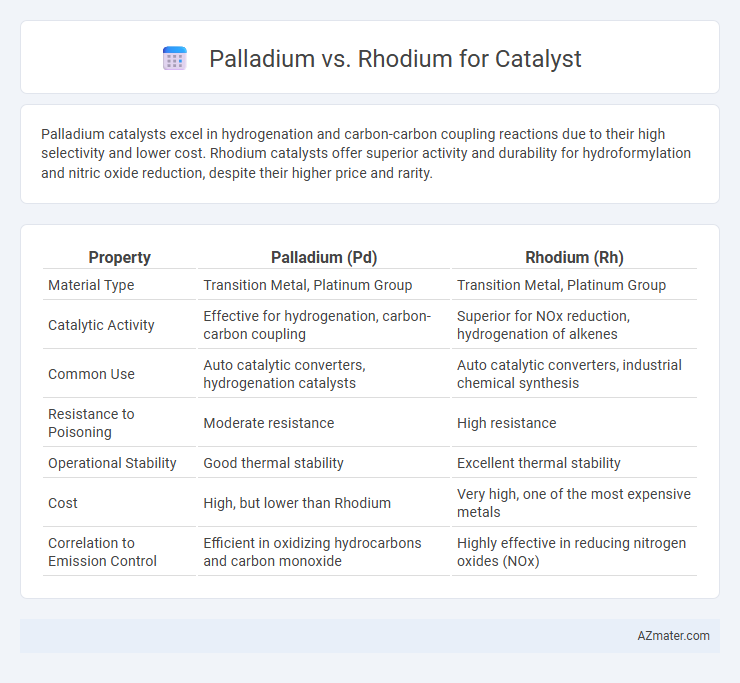
Palladium catalysts excel in hydrogenation and carbon-carbon coupling reactions due to their high selectivity and lower cost. Rhodium catalysts offer superior activity and durability for hydroformylation and nitric oxide reduction, despite their higher price and rarity.
Table of Comparison
| Property | Palladium (Pd) | Rhodium (Rh) |
|---|---|---|
| Material Type | Transition Metal, Platinum Group | Transition Metal, Platinum Group |
| Catalytic Activity | Effective for hydrogenation, carbon-carbon coupling | Superior for NOx reduction, hydrogenation of alkenes |
| Common Use | Auto catalytic converters, hydrogenation catalysts | Auto catalytic converters, industrial chemical synthesis |
| Resistance to Poisoning | Moderate resistance | High resistance |
| Operational Stability | Good thermal stability | Excellent thermal stability |
| Cost | High, but lower than Rhodium | Very high, one of the most expensive metals |
| Correlation to Emission Control | Efficient in oxidizing hydrocarbons and carbon monoxide | Highly effective in reducing nitrogen oxides (NOx) |
Introduction to Palladium and Rhodium in Catalysis
Palladium and rhodium are critical platinum-group metals widely used as catalysts in automotive emission control, with palladium excelling in oxidizing hydrocarbons and carbon monoxide. Rhodium is particularly effective for reducing nitrogen oxides due to its high resistance to sintering and ability to operate at high temperatures. Their complementary catalytic properties make them essential in three-way catalytic converters for controlling air pollution.
Chemical and Physical Properties Comparison
Palladium and rhodium are both platinum-group metals used as catalysts, with palladium having a melting point of 1555degC and rhodium melting at 1964degC, indicating higher thermal stability for rhodium. Chemically, palladium exhibits excellent hydrogen absorption and is highly effective in hydrogenation reactions, while rhodium is more resistant to oxidation and excels in selective catalytic reduction (SCR) processes. Physically, rhodium's higher hardness and corrosion resistance make it preferable for harsh environments whereas palladium's superior ductility facilitates easier coating and alloy formation in catalytic converters.
Mechanisms of Catalytic Action
Palladium facilitates hydrogenation and carbon-carbon coupling reactions through oxidative addition and reductive elimination, enabling smooth activation of substrates by forming Pd(0)/Pd(II) cycles. Rhodium exhibits exceptional activity in hydrogenation and hydroformylation by stabilizing key intermediates via Rh(I)/Rh(III) redox cycles, promoting selective substrate transformation. The differences in electronic structure and coordination environment influence their catalytic cycle mechanisms, resulting in distinct reactivity and selectivity profiles for various industrial applications.
Industrial Applications of Palladium and Rhodium
Palladium and rhodium are critical catalysts in industrial applications, particularly in automotive catalytic converters where palladium facilitates efficient oxidation of hydrocarbons and carbon monoxide, reducing harmful emissions. Rhodium excels in nitrogen oxide (NOx) reduction due to its superior resistance to high temperatures and corrosive environments, making it indispensable in automotive and chemical manufacturing industries. Both metals are also employed in hydrogenation and dehydrogenation processes within petrochemical refineries, with palladium favored for moderate reaction conditions and rhodium preferred for its robustness in severe environments.
Cost and Market Availability Analysis
Palladium and rhodium serve as critical catalysts in automotive and chemical industries, with palladium typically commanding a lower price per ounce than rhodium, whose market price can be two to three times higher due to its rarity and superior catalytic efficiency. The global supply of palladium benefits from stable production in Russia and South Africa, contributing to greater market availability, whereas rhodium's supply is more constrained, largely a by-product of platinum and nickel mining, leading to frequent price volatility. Cost-sensitive applications often favor palladium due to its relative abundance and lower expense, while rhodium is preferred when exceptional catalytic performance justifies its premium and limited availability.
Efficiency and Selectivity in Reactions
Palladium catalysts exhibit high efficiency in cross-coupling and hydrogenation reactions due to their excellent ability to activate C-C and C-H bonds with notable selectivity towards desired products. Rhodium catalysts offer superior selectivity in hydroformylation and asymmetric hydrogenation, providing high enantioselectivity and regioselectivity crucial for pharmaceutical intermediates. Comparing efficiency, palladium generally accelerates reaction rates effectively in carbon-carbon bond formation, while rhodium's precision in selective transformations reduces byproducts and enhances overall reaction sustainability.
Environmental Impact and Sustainability
Palladium catalysts often demand less energy for activation and exhibit lower toxicity compared to rhodium, supporting more eco-friendly industrial processes. Rhodium, while highly effective for reducing nitrogen oxides (NOx) emissions in automotive catalysts, is significantly rarer and more expensive, raising concerns about resource sustainability. Sustainable catalyst design increasingly favors palladium due to its balance of catalytic efficiency, lower environmental toxicity, and more abundant reserves, which contribute to reduced ecological footprint and long-term availability.
Longevity and Reusability in Catalysts
Palladium catalysts exhibit high longevity due to their resistance to deactivation and ability to maintain catalytic activity over multiple cycles, making them suitable for long-term industrial applications. Rhodium catalysts offer superior reusability in harsh reaction conditions, particularly in processes involving hydrogenation and hydroformylation, where their robustness prevents rapid degradation. Comparing both, palladium tends to excel in extended catalytic lifespan, while rhodium provides enhanced performance stability and consistent reusability under demanding chemical environments.
Recent Innovations and Research Trends
Recent innovations in palladium catalysts emphasize enhanced selectivity and stability in cross-coupling reactions, leveraging nanoparticle supports and ligand design for improved efficiency. Research trends in rhodium catalysis focus on asymmetric hydrogenation and C-H activation, utilizing chiral ligands and novel cyclometalated complexes to boost enantioselectivity and reaction scope. Advances in both metals incorporate sustainable practices, such as earth-abundant metal substitution and recyclable catalyst systems, aiming to reduce environmental impact while maintaining high catalytic performance.
Choosing the Right Metal for Catalytic Processes
Palladium and rhodium serve distinct yet complementary roles in catalytic processes, with palladium excelling in hydrogenation reactions due to its high activity and selectivity, while rhodium is preferred for reactions requiring robust resistance to poisoning and high-temperature stability, such as in automotive catalytic converters. Selecting the appropriate metal depends on specific reaction conditions, desired product selectivity, and catalyst longevity, where palladium's versatility suits fine chemical synthesis and rhodium's durability meets rigorous industrial demands. Optimal catalyst performance is achieved by balancing catalytic efficiency, cost-effectiveness, and environmental impact tailored to the targeted chemical transformation.
Infographic: Palladium vs Rhodium for Catalyst
 azmater.com
azmater.com