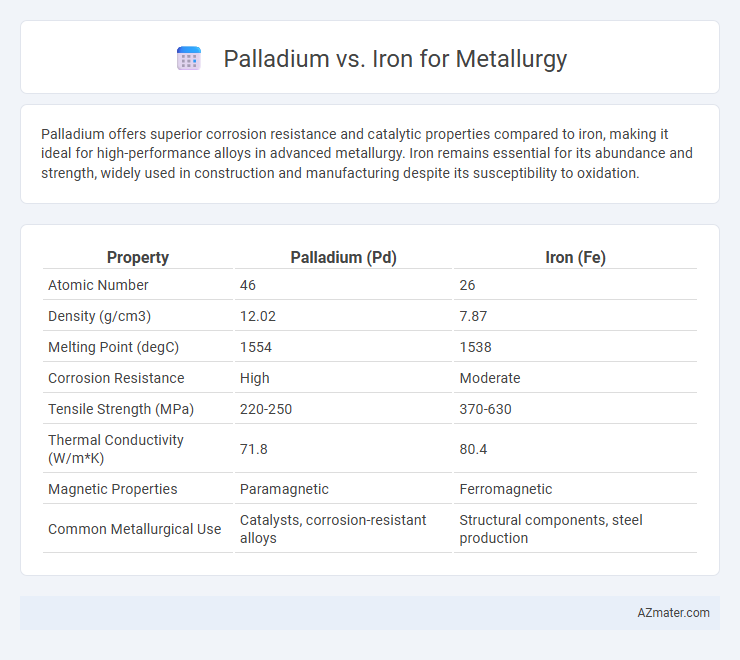
Palladium offers superior corrosion resistance and catalytic properties compared to iron, making it ideal for high-performance alloys in advanced metallurgy. Iron remains essential for its abundance and strength, widely used in construction and manufacturing despite its susceptibility to oxidation.
Table of Comparison
| Property | Palladium (Pd) | Iron (Fe) |
|---|---|---|
| Atomic Number | 46 | 26 |
| Density (g/cm3) | 12.02 | 7.87 |
| Melting Point (degC) | 1554 | 1538 |
| Corrosion Resistance | High | Moderate |
| Tensile Strength (MPa) | 220-250 | 370-630 |
| Thermal Conductivity (W/m*K) | 71.8 | 80.4 |
| Magnetic Properties | Paramagnetic | Ferromagnetic |
| Common Metallurgical Use | Catalysts, corrosion-resistant alloys | Structural components, steel production |
Introduction to Palladium and Iron in Metallurgy
Palladium and iron serve distinct roles in metallurgy due to their unique properties and applications. Palladium is a precious metal known for its excellent catalytic abilities, corrosion resistance, and high melting point, making it valuable in specialized alloy production and catalytic converters. Iron, abundant and versatile, is fundamental in steelmaking and structural applications, prized for its strength, ductility, and magnetic properties.
Elemental Properties: Palladium vs Iron
Palladium, a precious metal with atomic number 46, exhibits exceptional corrosion resistance, high catalytic activity, and excellent electrical conductivity, making it ideal for specialized metallurgical applications. Iron, atomic number 26, is abundant and valued for its magnetic properties, high tensile strength, and versatility in steel production, but it is prone to oxidation and rust. The elemental properties of palladium, such as its lower density (12.02 g/cm3) and higher melting point (1555degC), contrast with iron's density (7.87 g/cm3) and melting point (1538degC), influencing their distinct roles in metallurgy.
Abundance and Availability in Nature
Iron is one of the most abundant elements in the Earth's crust, comprising about 5% by weight, making it widely available and cost-effective for metallurgical applications. Palladium, a rare platinum-group metal, occurs in much smaller concentrations, typically found in nickel and copper ores, which limits its natural availability and increases its cost. The scarcity of palladium compared to iron significantly impacts its use, often reserving it for specialized applications requiring corrosion resistance or catalytic properties.
Mechanical Strength and Durability
Palladium exhibits superior corrosion resistance and durability compared to iron, making it ideal for applications requiring long-term stability under harsh conditions. While iron offers higher tensile strength and is more cost-effective for structural uses, palladium's mechanical strength is enhanced by its resistance to wear and fatigue. Metallurgical alloys combining palladium with iron can optimize both durability and mechanical performance, balancing cost and functionality effectively.
Corrosion Resistance Comparison
Palladium exhibits superior corrosion resistance compared to iron, as it forms a stable, inert oxide layer that prevents further oxidation, making it highly resistant to tarnishing and chemical attack in harsh environments. Iron, although widely used in metallurgy, is prone to rusting when exposed to oxygen and moisture, leading to the formation of iron oxides that degrade structural integrity. The inherent corrosion resistance of palladium enhances its suitability for applications in catalytic converters and electronic components, outperforming iron in longevity and maintenance requirements.
Applications in Modern Metallurgical Processes
Palladium is highly valued in modern metallurgy for its exceptional catalytic properties and resistance to corrosion, making it ideal for use in catalytic converters and hydrogen purification. Iron, a fundamental metal in metallurgy, is extensively utilized in steel production due to its strength, abundance, and versatility in alloys. The distinct applications of palladium and iron highlight their importance in specialized and large-scale industrial metallurgical processes.
Cost Effectiveness and Market Dynamics
Palladium, though highly effective in catalytic applications, remains significantly more expensive than iron due to its rarity and limited global supply, impacting cost-effectiveness in large-scale metallurgy projects. Iron's abundant availability and lower extraction costs make it the preferred metal for widespread industrial use despite palladium's superior corrosion resistance and catalytic properties. Market dynamics show palladium prices are influenced heavily by automotive demand and geopolitical factors, whereas iron's value is steadier, driven mainly by construction and infrastructure developments.
Environmental Impact and Sustainability
Palladium exhibits low environmental impact due to its high catalytic efficiency and recyclability in automotive and industrial applications, reducing harmful emissions significantly compared to iron. Iron mining and processing generate substantial carbon emissions and habitat disruption, posing challenges for sustainable metallurgy. Advances in palladium recovery and circular economy practices enhance its sustainability profile relative to the high-volume, energy-intensive extraction of iron.
Innovations and Emerging Technologies
Palladium in metallurgy offers superior catalytic properties and corrosion resistance, driving innovations in hydrogen storage and fuel cell technologies. Iron, abundant and cost-effective, remains critical for developing advanced steel alloys with enhanced strength and lightweight characteristics through emerging additive manufacturing techniques. Advances in nano-engineering and surface treatments further enhance the performance of palladium and iron-based materials, expanding their applications in sustainable energy and industrial processes.
Choosing Between Palladium and Iron for Specific Uses
Palladium offers superior corrosion resistance and excellent catalytic properties, making it ideal for electronics, automotive catalytic converters, and dental alloys, whereas iron provides mechanical strength, cost-efficiency, and magnetic properties suited for structural components and heavy machinery. The choice depends on application requirements: palladium excels in environments demanding chemical stability and conductivity, while iron is preferred for load-bearing and magnetic applications due to its abundance and strength. Metallurgical selection balances factors such as cost, durability, and functionality based on the specific operational conditions.
Infographic: Palladium vs Iron for Metallurgy
 azmater.com
azmater.com