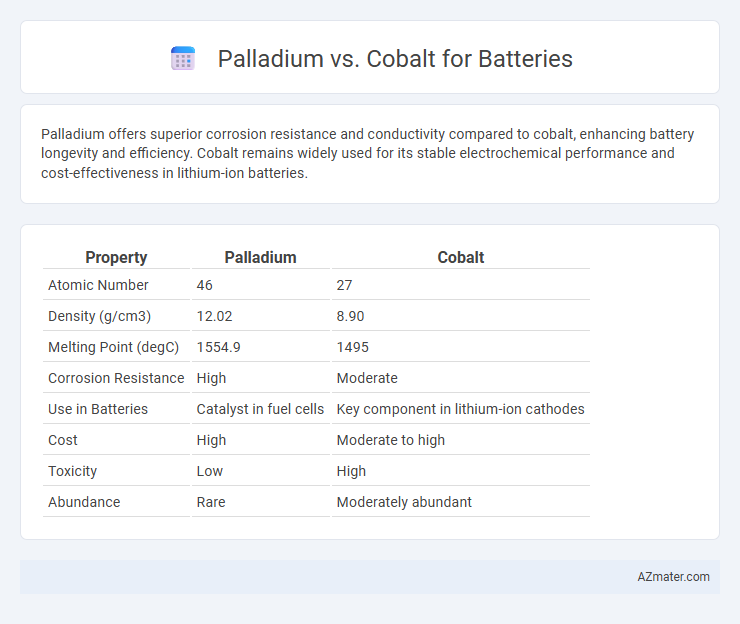
Palladium offers superior corrosion resistance and conductivity compared to cobalt, enhancing battery longevity and efficiency. Cobalt remains widely used for its stable electrochemical performance and cost-effectiveness in lithium-ion batteries.
Table of Comparison
| Property | Palladium | Cobalt |
|---|---|---|
| Atomic Number | 46 | 27 |
| Density (g/cm3) | 12.02 | 8.90 |
| Melting Point (degC) | 1554.9 | 1495 |
| Corrosion Resistance | High | Moderate |
| Use in Batteries | Catalyst in fuel cells | Key component in lithium-ion cathodes |
| Cost | High | Moderate to high |
| Toxicity | Low | High |
| Abundance | Rare | Moderately abundant |
Introduction to Palladium and Cobalt in Battery Technology
Palladium and cobalt play distinct roles in battery technology, with cobalt being a critical component in lithium-ion batteries for enhancing energy density and stability, while palladium is primarily used in catalyst applications within fuel cells. Cobalt's electrochemical properties improve battery lifespan and charge efficiency, making it essential for electric vehicle batteries. Palladium, though less common in traditional batteries, contributes to advancing fuel cell technology by facilitating hydrogen oxidation reactions.
Chemical Properties and Behavior in Batteries
Palladium exhibits excellent catalytic properties and high corrosion resistance, enhancing battery efficiency by promoting faster charge-discharge cycles and stability in electrode reactions. Cobalt offers superior electrochemical performance with high energy density and structural stability, commonly used in lithium-ion batteries for its ability to maintain capacity and support long cycle life. While palladium is more expensive and less abundant, its unique catalytic behavior can improve battery kinetics, whereas cobalt remains favored for maintaining battery performance under varied operating conditions.
Energy Density Comparison
Palladium batteries exhibit higher energy density compared to cobalt-based batteries, often reaching up to 250 Wh/kg, which enhances electric vehicle range and device runtime. Cobalt's energy density typically ranges around 180-200 Wh/kg, but its supply chain volatility and ethical sourcing issues limit scalability. The superior electrochemical properties of palladium enable longer cycle life and greater energy storage efficiency, making it a promising alternative in advanced battery technologies.
Cost and Market Availability
Palladium is significantly more expensive than cobalt, with prices often exceeding $2,000 per ounce compared to cobalt's approximate $30 per pound, impacting battery manufacturing costs. Market availability of cobalt is more stable due to its widespread mining in the Democratic Republic of Congo and other countries, whereas palladium supply is limited, primarily sourced from Russia and South Africa, leading to potential supply risks. The cost-effectiveness and larger-scale availability of cobalt make it a preferred choice for mass-market battery production despite palladium's favorable catalytic properties.
Performance in Rechargeable Batteries
Palladium offers superior catalytic performance and higher electrical conductivity compared to cobalt, enhancing battery charge efficiency and lifespan in rechargeable batteries. Cobalt, widely used in lithium-ion batteries, provides stable energy density and thermal stability but faces limitations in rate capability and sustainability. Advances in palladium-based electrode materials show promise for improving fast-charging rates and cycle stability, positioning palladium as a potential alternative to cobalt in next-generation energy storage technologies.
Environmental Impact and Sustainability
Palladium and cobalt both play critical roles in battery technology, but cobalt's environmental impact is more severe due to extensive mining activities in the Congo, leading to habitat destruction and human rights concerns. Palladium, primarily sourced as a byproduct of platinum and nickel mining, poses fewer direct environmental risks, contributing to improved sustainability in battery production. Innovations in recycling and alternative materials aim to reduce cobalt dependency, promoting greener and more ethical battery solutions.
Safety and Stability in Battery Applications
Palladium offers superior chemical stability and resistance to corrosion compared to cobalt, making it a safer choice for battery applications with reduced risk of thermal runaway and fire hazards. Batteries using palladium exhibit enhanced cycle life and consistent performance under high-temperature conditions, improving overall operational safety. In contrast, cobalt-based batteries face challenges such as toxicity and instability under extreme conditions, which can compromise safety and longevity.
Recycling and Lifecycle Management
Palladium and cobalt serve distinct roles in battery technology, with cobalt primarily used in lithium-ion cathodes and palladium more common in fuel cells and battery catalysts. Cobalt recycling benefits from established industrial processes, enabling efficient recovery from spent batteries, which mitigates supply risks and environmental impact. Palladium recycling remains less widespread but is critical due to its high value, and innovations in lifecycle management aim to improve reclamation efficiency and reduce reliance on primary mining.
Industry Adoption and Future Trends
Palladium and cobalt play distinct roles in battery technology, with cobalt widely adopted in lithium-ion batteries for electric vehicles due to its energy density and stability, despite supply chain concerns and ethical sourcing issues. Palladium, primarily used as a catalytic agent, is emerging in research for next-generation batteries but has yet to see significant industrial adoption compared to cobalt's critical role. Future trends indicate a shift towards reducing cobalt dependency through alternative materials and recycling innovations, while palladium could gain traction as its catalytic properties are leveraged in advanced battery chemistries.
Conclusion: Choosing Between Palladium and Cobalt
Palladium offers excellent catalytic properties and corrosion resistance, making it effective for enhancing battery efficiency and longevity, while cobalt provides high energy density and thermal stability crucial for current lithium-ion batteries. Selecting between palladium and cobalt depends on factors such as cost, availability, environmental impact, and specific battery performance requirements. Emerging research trends suggest palladium may become more viable with improved recycling and reduced reliance on cobalt, which faces supply chain and ethical challenges.
Infographic: Palladium vs Cobalt for Battery
 azmater.com
azmater.com