Osmium, a dense and rare metal, is unsuitable for fireworks due to its high toxicity and cost, whereas Strontium is widely used for producing vibrant red flames in fireworks because of its strong emission spectrum and relative safety. Strontium compounds enhance color intensity and stability, making them a preferred material in pyrotechnic formulations.
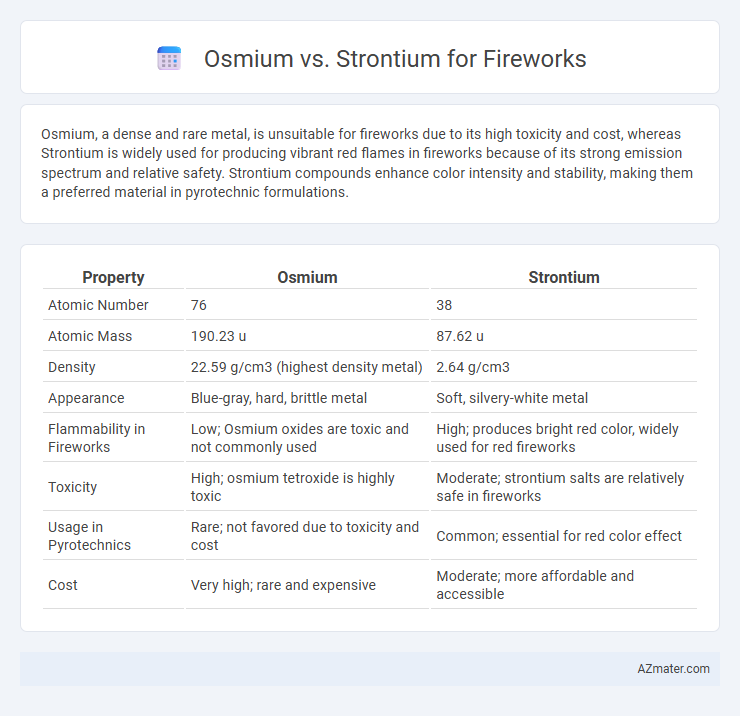
Table of Comparison
| Property | Osmium | Strontium |
|---|---|---|
| Atomic Number | 76 | 38 |
| Atomic Mass | 190.23 u | 87.62 u |
| Density | 22.59 g/cm3 (highest density metal) | 2.64 g/cm3 |
| Appearance | Blue-gray, hard, brittle metal | Soft, silvery-white metal |
| Flammability in Fireworks | Low; Osmium oxides are toxic and not commonly used | High; produces bright red color, widely used for red fireworks |
| Toxicity | High; osmium tetroxide is highly toxic | Moderate; strontium salts are relatively safe in fireworks |
| Usage in Pyrotechnics | Rare; not favored due to toxicity and cost | Common; essential for red color effect |
| Cost | Very high; rare and expensive | Moderate; more affordable and accessible |
Introduction to Osmium and Strontium in Fireworks
Osmium, a dense and rare transition metal, is seldom used in fireworks due to its toxicity and high cost, but it offers unique blue-gray hues when burned. Strontium, a more common alkaline earth metal, is widely utilized in pyrotechnics for producing vivid red flames and stable combustion. The contrasting chemical properties of osmium and strontium define their distinct roles in firework compositions, with strontium dominating for color effects and osmium being largely experimental or specialty.
Chemical Properties Relevant to Pyrotechnics
Osmium's high density and oxidation state versatility make it less favorable for fireworks due to its limited light emission and high toxicity. Strontium offers vibrant red hues in pyrotechnics, attributed to its strontium salts' efficient electron excitation and emission in the visible spectrum. The reactivity of strontium with oxidizers enables controlled combustion, essential for color intensity and stability in fireworks displays.
Color Effects Produced by Osmium and Strontium
Osmium produces unique spark effects with a subtle bluish-white glow in fireworks, offering a rare metallic shimmer that enhances visual complexity. Strontium is widely used for its bright red flame color, delivering vivid and intense hues ideal for creating striking contrast in pyrotechnic displays. The combination of osmium's metallic sparks and strontium's vibrant red coloration enables fireworks to achieve richer and more diverse visual effects.
Availability and Cost Comparison
Osmium, a dense and rare metal, is significantly less available and considerably more expensive than strontium, which is more abundant and widely used in fireworks for its vibrant red color. The high cost and scarcity of osmium make it impractical for large-scale fireworks production compared to strontium, which is preferred for economic and supply reliability reasons. Strontium compounds are readily sourced from various minerals and produced in larger quantities, leading to a lower price point suitable for commercial pyrotechnics.
Safety and Handling Considerations
Osmium, a dense and rare metal, poses significant safety risks in fireworks due to its high toxicity and potential to form volatile osmium tetroxide, which is harmful when inhaled. Strontium compounds, widely used to produce vibrant red colors, are relatively safer but still require careful handling due to their moderate toxicity and potential environmental impact. Proper storage, use of personal protective equipment, and adherence to regulatory guidelines are essential when working with either element to minimize health hazards and ensure safe fireworks manufacturing.
Environmental Impact of Both Elements
Osmium and strontium differ significantly in their environmental impact when used in fireworks; osmium compounds are highly toxic and can release harmful osmium tetroxide vapors that pose risks to air quality and human health. Strontium, commonly used to produce red colors in pyrotechnics, presents lower toxicity but can contribute to soil and water contamination due to its chemical persistence after fireworks discharge. Environmental assessments favor strontium over osmium for firework applications due to strontium's comparatively safer ecological profile and reduced health hazards.
Performance in Pyrotechnic Compositions
Osmium exhibits limited use in pyrotechnic compositions due to its high density and low flammability, resulting in minimal contribution to color or explosive effects. Strontium compounds, particularly strontium nitrate and strontium carbonate, are preferred for their vibrant red flame coloration and strong oxidizing properties enhancing combustion efficiency. The superior luminous intensity and consistent color yield of strontium-based mixtures significantly outperform osmium in fireworks performance metrics.
Common Uses in Commercial Fireworks
Strontium compounds, particularly strontium nitrate and strontium carbonate, are widely used in commercial fireworks to produce vibrant red colors and enhance flame stability. Osmium is rarely used due to its high cost and limited color contribution, making it impractical for large-scale fireworks manufacturing. The dominance of strontium in pyrotechnics is attributed to its effective red coloration and availability, which are critical for visually striking firework displays.
Regulatory Issues and Legal Restrictions
Osmium is rarely used in fireworks due to its scarcity, high toxicity, and stringent regulatory controls on heavy metals to prevent environmental contamination and health hazards. Strontium, favored for its vibrant red flame, faces strict regulations related to its soluble compounds to avoid water pollution and soil toxicity, governed by agencies like the EPA and REACH. Both elements require compliance with hazardous material transportation laws and safe handling protocols, significantly impacting their availability and usage in the pyrotechnic industry.
Future Trends and Innovations in Firework Chemistry
Future trends in firework chemistry emphasize the use of less toxic and more environmentally friendly elements, reducing reliance on traditionally hazardous metals like osmium, which is rare and expensive. Strontium continues to gain prominence due to its strong red emission and relative abundance, supporting innovations in safer, more vibrant color formulations. Emerging technologies explore nanoengineering of strontium compounds to enhance color intensity and flame stability, paving the way for more sustainable and visually striking pyrotechnics.
Infographic: Osmium vs Strontium for Firework
 azmater.com
azmater.com