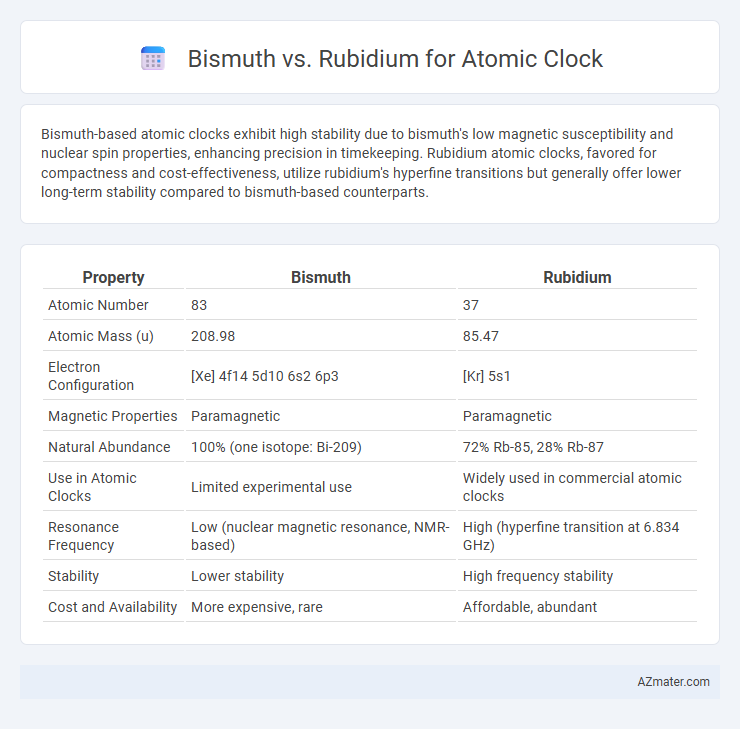
Bismuth-based atomic clocks exhibit high stability due to bismuth's low magnetic susceptibility and nuclear spin properties, enhancing precision in timekeeping. Rubidium atomic clocks, favored for compactness and cost-effectiveness, utilize rubidium's hyperfine transitions but generally offer lower long-term stability compared to bismuth-based counterparts.
Table of Comparison
| Property | Bismuth | Rubidium |
|---|---|---|
| Atomic Number | 83 | 37 |
| Atomic Mass (u) | 208.98 | 85.47 |
| Electron Configuration | [Xe] 4f14 5d10 6s2 6p3 | [Kr] 5s1 |
| Magnetic Properties | Paramagnetic | Paramagnetic |
| Natural Abundance | 100% (one isotope: Bi-209) | 72% Rb-85, 28% Rb-87 |
| Use in Atomic Clocks | Limited experimental use | Widely used in commercial atomic clocks |
| Resonance Frequency | Low (nuclear magnetic resonance, NMR-based) | High (hyperfine transition at 6.834 GHz) |
| Stability | Lower stability | High frequency stability |
| Cost and Availability | More expensive, rare | Affordable, abundant |
Introduction to Atomic Clocks
Atomic clocks use the precise frequency of electromagnetic radiation emitted or absorbed by atoms, with rubidium and bismuth being notable choices due to their atomic properties. Rubidium atomic clocks are widely favored for their stability and affordability, relying on the hyperfine transitions of rubidium-87 atoms to maintain accurate timekeeping. Bismuth, with its unique nuclear spin characteristics, offers alternative potential but remains less common in commercial atomic clock applications compared to rubidium.
Overview of Bismuth and Rubidium Elements
Bismuth is a heavy post-transition metal with atomic number 83, known for its high stability and low toxicity, making it suitable for precise atomic clock applications due to its distinct hyperfine structure. Rubidium, an alkali metal with atomic number 37, is widely used in atomic clocks for its favorable ground-state hyperfine splitting that enables highly accurate frequency standards. Both elements offer unique quantum properties, but rubidium's ease of optical pumping and well-studied transitions often give it an edge in commercial atomic clock technology.
Atomic Structure: Bismuth vs Rubidium
Bismuth features a complex atomic structure with 83 protons and a large number of electrons, resulting in multiple hyperfine transitions that complicate its use in atomic clocks. Rubidium, with 37 protons and a simpler electron configuration, provides a more stable and easily accessible hyperfine transition ideal for precision timekeeping. The rubidium atomic clock leverages the 5S1/2 ground state hyperfine splitting, offering consistent frequency signals compared to bismuth's more intricate atomic structure.
Hyperfine Transitions Relevant to Timekeeping
Bismuth and rubidium atomic clocks differ significantly in their hyperfine transitions, which are critical for precision timekeeping. Rubidium-87 clocks utilize the 6.834 GHz hyperfine transition between the ground state levels, offering stability and compact design favored in commercial and GPS applications. Bismuth, with its more complex electron configuration and higher nuclear spin, presents hyperfine transitions that are less commonly exploited but show potential for improved frequency stability in advanced atomic clock research.
Stability and Accuracy in Atomic Clocks
Bismuth atomic clocks offer enhanced stability due to their lower magnetic susceptibility and reduced environmental perturbations compared to rubidium, which contributes to prolonged coherence times. Rubidium atomic clocks provide high accuracy through well-established hyperfine transition frequencies, but their performance is more susceptible to temperature fluctuations and magnetic field variations. The superior stability of bismuth can potentially lead to greater long-term accuracy in timekeeping applications, making it a promising candidate for next-generation atomic clocks.
Practical Implementation: Bismuth vs Rubidium
Bismuth atomic clocks offer higher frequency stability due to their complex hyperfine structures but face challenges in miniaturization and cost compared to rubidium clocks. Rubidium atomic clocks are widely used for practical implementation because of their compact size, lower power consumption, and cost-effective manufacturing, making them ideal for commercial and military applications. Integration of rubidium clocks in GPS and communication systems highlights their robustness and reliability in real-world environments.
Environmental Sensitivity and Interference
Bismuth atomic clocks exhibit lower environmental sensitivity due to bismuth's stable nuclear spin and reduced susceptibility to magnetic field fluctuations, enhancing timekeeping accuracy in varied conditions. In contrast, rubidium clocks, while cost-effective and widely used, experience greater interference from temperature variations and magnetic fields, leading to frequency drift. Optimizing atomic clock performance requires balancing bismuth's resilience against environmental perturbations with rubidium's practical deployment advantages.
Cost and Scalability Considerations
Bismuth offers lower material costs and greater abundance compared to rubidium, making it a more economical choice for atomic clock development. Rubidium, while more expensive and less abundant, benefits from established manufacturing processes that support large-scale production and integration. Scalability favors rubidium for commercial atomic clocks due to mature supply chains, whereas bismuth-based clocks may achieve cost advantages in niche or emerging applications with optimized fabrication techniques.
Recent Research and Technological Advances
Recent research indicates bismuth-based atomic clocks demonstrate enhanced stability and reduced magnetic sensitivity compared to rubidium clocks, which suffer from higher quantum noise levels. Technological advances in bismuth vapor cell fabrication and laser stabilization have improved frequency precision to the 10^-15 range, surpassing traditional rubidium clock performances. Innovations in coherent population trapping techniques enable bismuth clocks to achieve longer coherence times, making them promising candidates for next-generation portable atomic clocks.
Future Prospects for Bismuth and Rubidium Atomic Clocks
Bismuth atomic clocks show promise for future advancements due to their potential for higher frequency stability and reduced environmental sensitivity compared to rubidium clocks. Rubidium atomic clocks remain widely used for their proven reliability and compact size, but ongoing research aims to enhance their long-term accuracy and integration in portable devices. Innovations in quantum technology and materials science are driving the development of both bismuth and rubidium clocks, targeting improved precision for telecommunications, space navigation, and fundamental scientific research.
Infographic: Bismuth vs Rubidium for Atomic clock
 azmater.com
azmater.com