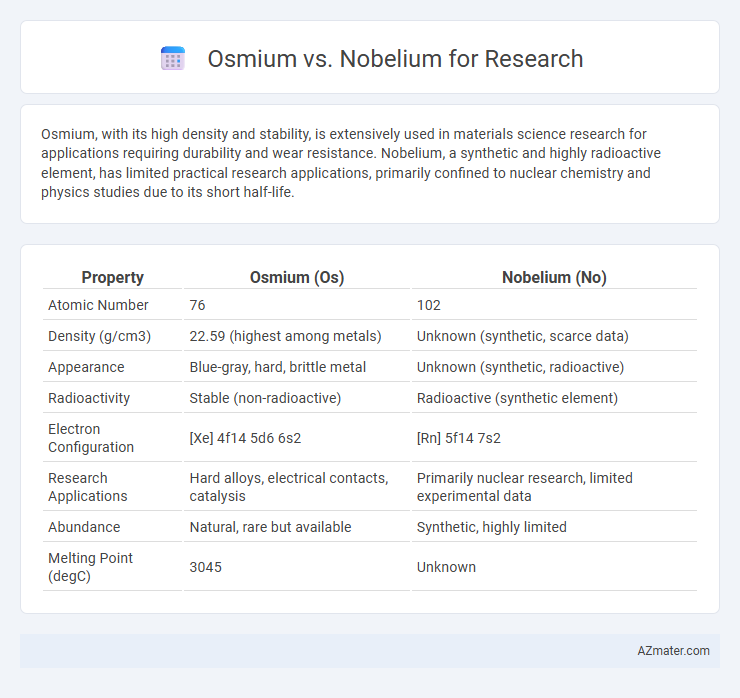
Osmium, with its high density and stability, is extensively used in materials science research for applications requiring durability and wear resistance. Nobelium, a synthetic and highly radioactive element, has limited practical research applications, primarily confined to nuclear chemistry and physics studies due to its short half-life.
Table of Comparison
| Property | Osmium (Os) | Nobelium (No) |
|---|---|---|
| Atomic Number | 76 | 102 |
| Density (g/cm3) | 22.59 (highest among metals) | Unknown (synthetic, scarce data) |
| Appearance | Blue-gray, hard, brittle metal | Unknown (synthetic, radioactive) |
| Radioactivity | Stable (non-radioactive) | Radioactive (synthetic element) |
| Electron Configuration | [Xe] 4f14 5d6 6s2 | [Rn] 5f14 7s2 |
| Research Applications | Hard alloys, electrical contacts, catalysis | Primarily nuclear research, limited experimental data |
| Abundance | Natural, rare but available | Synthetic, highly limited |
| Melting Point (degC) | 3045 | Unknown |
Introduction to Osmium and Nobelium
Osmium is a dense, bluish-white transition metal known for its hardness and high melting point, widely utilized in specialized scientific instruments and catalytic applications due to its extreme durability. Nobelium, a synthetic radioactive actinide, is produced in minute quantities through nuclear reactions and primarily studied for its complex nuclear properties and placement in the actinide series. Research involving osmium emphasizes its practical chemical stability and industrial uses, while nobelium research concentrates on its nuclear behavior and rare-earth element characteristics.
Atomic Structure and Properties Comparison
Osmium exhibits a dense atomic structure with an atomic number of 76 and a high electron density primarily in its 5d orbitals, contributing to its exceptional hardness and high melting point of 3033degC. Nobelium, with atomic number 102, is a synthetic actinide element characterized by a more complex electron configuration involving 5f orbitals, resulting in high radioactivity and limited stability. The fundamental differences in electron shell configuration between osmium's transition metal nature and nobelium's actinide properties significantly influence their chemical reactivity and suitability for specific research applications.
Occurrence and Availability in Nature
Osmium, a rare transition metal found primarily in platinum ores, occurs naturally in the Earth's crust at approximately 0.0013 ppm, making it one of the densest and scarcest stable elements readily available for research. Nobelium, an artificially synthesized radioisotope with no stable isotopes, does not occur naturally and is produced in particle accelerators in extremely limited quantities, thus significantly restricting its availability for experimental studies. The natural occurrence and accessibility of osmium facilitate comprehensive material science research, while nobelium research is confined to specialized nuclear chemistry and physics investigations due to its synthetic origin and rapid decay.
Synthesis Methods and Production Challenges
Osmium is mainly produced through the extraction and refining of platinum ores, involving high-temperature processes and complex chemical separation techniques to isolate this dense, rare transition metal. Nobelium synthesis is achieved exclusively through particle accelerator methods, where heavy ion collisions create this highly unstable synthetic element, requiring specialized facilities and short detection times due to its rapid decay. Challenges in osmium production include its rarity and toxicity, while nobelium presents difficulties in yield, stability, and the need for advanced nuclear reactors or accelerators.
Chemical Reactivity and Stability
Osmium exhibits high chemical stability with a dense, corrosion-resistant nature, making it suitable for research requiring durable materials and catalysts. Nobelium, a synthetic actinide with a short half-life and high radioactivity, shows limited chemical reactivity and is primarily studied for nuclear and radiochemical properties rather than conventional chemical stability. Research applications favor osmium for stable, long-term experiments, while nobelium's instability restricts its use to specialized investigations in nuclear chemistry.
Uses of Osmium in Scientific Research
Osmium is extensively used in scientific research due to its exceptional density and hardness, making it ideal for electron microscopy as a staining agent to improve image resolution. It serves crucial roles in organic chemistry as a catalyst for oxidation reactions and in material science for creating durable alloys and specialized electrical contacts. Nobelium, a synthetic and highly radioactive element, has limited research applications primarily confined to nuclear physics experiments and has no significant use in practical scientific research.
Nobelium Applications in Modern Research
Nobelium, a synthetic element with atomic number 102, plays a critical role in nuclear physics and chemistry research due to its radioactive properties and position in the actinide series. Its applications include studying nuclear reactions, synthesis of heavier elements, and probing the stability of superheavy nuclei, which are pivotal in understanding atomic structure and nuclear forces. Osmium, though valuable for its density and catalytic properties, lacks the unique radioactive characteristics that make Nobelium indispensable in advanced nuclear research.
Safety and Handling Considerations
Osmium, a dense transition metal, requires careful handling due to its toxic osmium tetroxide, which forms on exposure to air, necessitating well-ventilated environments and protective equipment in research settings. Nobelium, a synthetic radioactive element with a short half-life, demands specialized radiation shielding and remote handling techniques to ensure researcher safety and prevent contamination. The contrasting chemical stability and radioactivity profiles of osmium and nobelium dictate distinct safety protocols, influencing their respective research applications and laboratory requirements.
Cost and Accessibility for Laboratories
Osmium, a dense and rare platinum-group metal, is relatively more accessible and cost-effective for laboratory research compared to Nobelium, a highly radioactive synthetic element with limited availability. Osmium's price remains significantly lower due to its natural abundance and established supply chains, facilitating broader experimental usage. In contrast, Nobelium's production involves complex nuclear reactors or particle accelerators, resulting in extremely high costs and minimal quantities, restricting its accessibility primarily to specialized nuclear research facilities.
Future Prospects in Elemental Research
Osmium's exceptional density and corrosion resistance make it a prime candidate for advanced material science and nanoengineering applications, offering durable components in extreme environments. Nobelium, a synthetic and highly radioactive actinide, presents significant challenges for research due to its short half-life but holds potential in expanding understanding of heavy element stability and nuclear chemistry. Future elemental research aims to harness osmium's unique physical properties for industrial innovation while exploring nobelium's nuclear characteristics to unlock insights into superheavy element synthesis.
Infographic: Osmium vs Nobelium for Research
 azmater.com
azmater.com