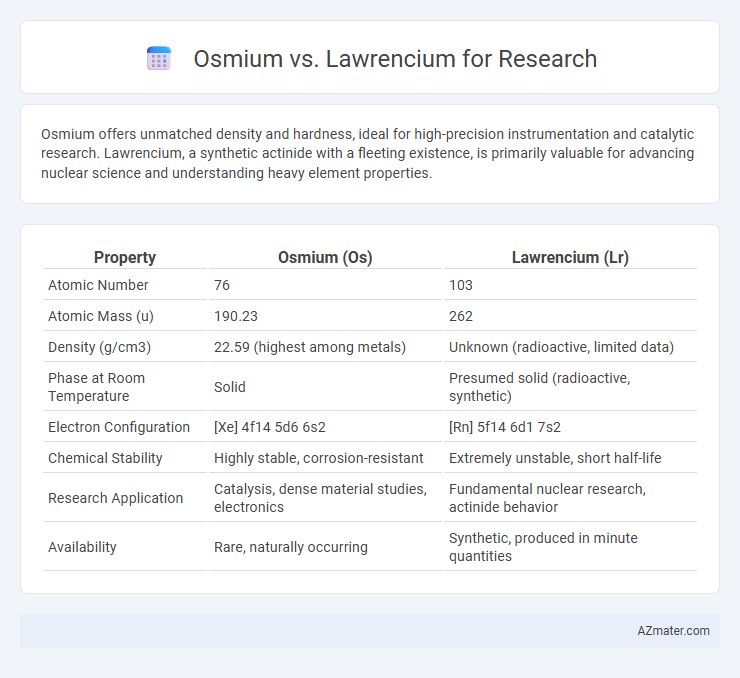
Osmium offers unmatched density and hardness, ideal for high-precision instrumentation and catalytic research. Lawrencium, a synthetic actinide with a fleeting existence, is primarily valuable for advancing nuclear science and understanding heavy element properties.
Table of Comparison
| Property | Osmium (Os) | Lawrencium (Lr) |
|---|---|---|
| Atomic Number | 76 | 103 |
| Atomic Mass (u) | 190.23 | 262 |
| Density (g/cm3) | 22.59 (highest among metals) | Unknown (radioactive, limited data) |
| Phase at Room Temperature | Solid | Presumed solid (radioactive, synthetic) |
| Electron Configuration | [Xe] 4f14 5d6 6s2 | [Rn] 5f14 6d1 7s2 |
| Chemical Stability | Highly stable, corrosion-resistant | Extremely unstable, short half-life |
| Research Application | Catalysis, dense material studies, electronics | Fundamental nuclear research, actinide behavior |
| Availability | Rare, naturally occurring | Synthetic, produced in minute quantities |
Introduction to Osmium and Lawrencium
Osmium, a dense transition metal with atomic number 76, is renowned for its hardness, high melting point, and applications in catalysis and electronics. Lawrencium, atomic number 103, belongs to the actinide series and is highly radioactive, with limited availability, making it primarily significant for nuclear research and theoretical chemistry. Comparing these elements highlights osmium's practical utility versus lawrencium's role in advanced scientific studies on heavy element behavior and atomic structure.
Elemental Properties Overview
Osmium exhibits exceptional density and hardness with a high melting point of 3,033degC, making it valuable for applications requiring durability and wear resistance in experimental research. Lawrencium, a synthetic element with atomic number 103, is highly unstable and radioactive, possessing no stable isotopes and limited practical use due to its short half-life. Research involving Lawrencium primarily focuses on exploring its electron configuration and placement within the actinide series, offering insights into heavy element chemistry and nuclear synthesis.
Availability and Natural Occurrence
Osmium is a naturally occurring element found primarily in platinum ores with an abundance of about 0.001 to 0.005 ppm in the Earth's crust, making it relatively more available for research purposes. Lawrencium, on the other hand, is a synthetic element produced only in particle accelerators, with no natural occurrence, resulting in extremely limited availability and only trace amounts generated for experimental studies. The accessibility of osmium supports its use in various scientific applications, while lawrencium remains primarily a subject of theoretical research due to its scarcity.
Atomic Structure Comparison
Osmium (Os) features a stable electron configuration of [Xe] 4f14 5d6 6s2, with a dense nucleus containing 76 protons, making it one of the heaviest stable transition metals ideal for materials research. Lawrencium (Lr), an actinide with atomic number 103, exhibits a more complex and less stable electron configuration [Rn] 5f14 7s2 7p1, complicating its study due to relativistic effects and short half-lives. The fundamental difference in atomic structure between Osmium and Lawrencium significantly affects their chemical properties and practical applications in research, with Osmium favored for its stability and Lawrencium for exploring superheavy element behaviors.
Chemical Reactivity and Behavior
Osmium exhibits high chemical reactivity characterized by its stable oxidation states, particularly +4 and +8, enabling diverse coordination chemistry and catalytic applications in research. Lawrencium, a synthetic actinide with an electron configuration resembling lutetium, displays limited observable chemical behavior due to its short half-life and scarce availability, but predictions suggest a +3 oxidation state dominance. Comparative studies emphasize osmium's practical utility in catalysis and material science, whereas lawrencium's research remains primarily theoretical, focusing on relativistic effects influencing heavy element chemistry.
Uses in Scientific Research
Osmium is extensively used in scientific research for its high density and hardness, making it ideal for applications in electron microscopy as a staining agent to enhance image contrast. Lawrencium, being a synthetic and highly radioactive element, primarily serves in nuclear research to study the properties of heavy elements and understand actinide behavior. While osmium plays a crucial role in material science and microscopy, lawrencium's uses are largely confined to experimental nuclear chemistry and atomic physics investigations.
Handling and Safety Considerations
Osmium, a dense transition metal with high toxicity due to its volatile osmium tetroxide compound, requires stringent safety measures including proper ventilation, protective gloves, and respiratory protection during handling. Lawrencium, a synthetic actinide with a short half-life and limited availability, poses radiological hazards necessitating specialized containment, minimal exposure time, and remote handling tools to protect researchers. Both elements demand rigorous protocols to mitigate chemical toxicity and radiological risks, impacting their practicality and safety in research applications.
Cost and Accessibility for Researchers
Osmium, a dense transition metal, is more readily available and cost-effective for research compared to Lawrencium, a synthetic element produced only in trace amounts via particle accelerators. The high cost and scarcity of Lawrencium limit its accessibility, confining its study primarily to specialized nuclear research facilities with advanced equipment. Researchers favor Osmium for experiments requiring stable elements with unique properties, while Lawrencium's rare isotopes and short half-life restrict practical applications.
Technological Applications
Osmium, known for its extreme density and hardness, is widely utilized in technological applications such as electrical contacts, filaments, and fountain pen nibs, offering exceptional durability and corrosion resistance. Lawrencium, a synthetic and highly radioactive element with no stable isotopes, has limited practical applications beyond scientific research, primarily in nuclear physics and chemistry for studying heavy element properties and nuclear reactions. The significant technological advantage of osmium lies in its material properties, whereas lawrencium's value remains predominantly experimental within advanced research laboratories.
Future Research Directions
Osmium, with its high density and unique catalytic properties, offers promising avenues for advanced material science and nanotechnology research, particularly in areas like fuel cells and superhard alloys. Lawrencium, as a synthetic element in the actinide series, remains largely unexplored due to its short half-life, but future research may unlock insights into relativistic effects and nuclear chemistry. Emerging experimental techniques and improved detection methods will be pivotal in expanding knowledge of lawrencium's chemical behavior and potential applications in fundamental physics.
Infographic: Osmium vs Lawrencium for Research
 azmater.com
azmater.com