Osmium, with its high density and corrosion resistance, is ideal for advanced material synthesis and industrial applications. Einsteinium, a rare and highly radioactive actinide, is primarily used for nuclear research and experimental synthesis of heavier elements.
Table of Comparison
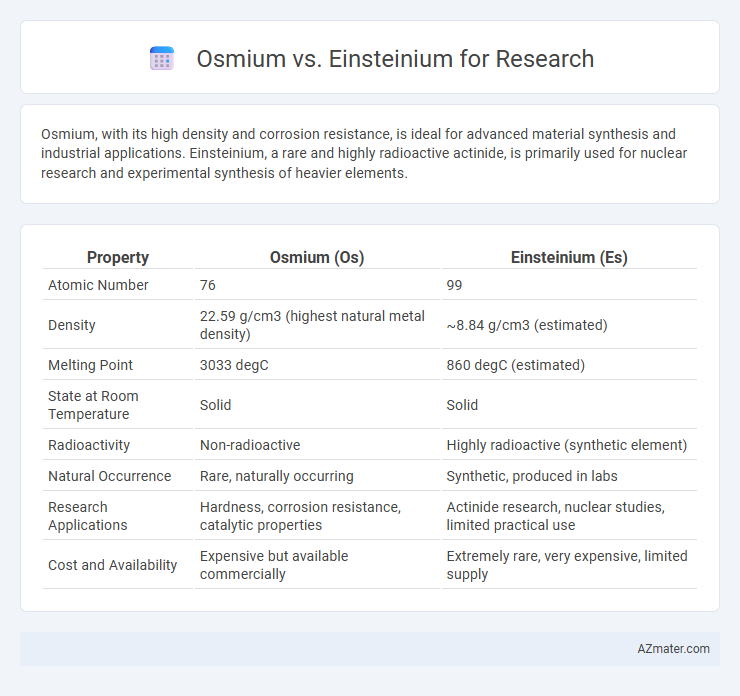
| Property | Osmium (Os) | Einsteinium (Es) |
|---|---|---|
| Atomic Number | 76 | 99 |
| Density | 22.59 g/cm3 (highest natural metal density) | ~8.84 g/cm3 (estimated) |
| Melting Point | 3033 degC | 860 degC (estimated) |
| State at Room Temperature | Solid | Solid |
| Radioactivity | Non-radioactive | Highly radioactive (synthetic element) |
| Natural Occurrence | Rare, naturally occurring | Synthetic, produced in labs |
| Research Applications | Hardness, corrosion resistance, catalytic properties | Actinide research, nuclear studies, limited practical use |
| Cost and Availability | Expensive but available commercially | Extremely rare, very expensive, limited supply |
Introduction to Osmium and Einsteinium
Osmium, a dense transition metal with atomic number 76, is prized in research for its high density, hardness, and resistance to corrosion, making it valuable in catalysis and material science studies. Einsteinium, a synthetic element with atomic number 99, is a member of the actinide series primarily produced in nuclear reactors, notable for its radioactivity and limited availability, which restricts its use mostly to fundamental nuclear research. Comparing osmium and einsteinium highlights their contrasting roles, with osmium facilitating practical applications in chemical and physical experiments while einsteinium provides insights into nuclear reactions and the properties of heavy elements.
Atomic Structure and Properties
Osmium, with atomic number 76, is a dense transition metal characterized by a stable electron configuration [Xe]4f^14 5d^6 6s^2, making it ideal for research requiring high-density materials and catalysis. Einsteinium, atomic number 99, is a radioactive actinide with an electron configuration of [Rn]5f^11 7s^2, notable for its synthetic nature and complexity in handling due to radioactivity, which limits experimental research. The contrasting atomic structures directly influence their chemical behaviors and applications, with osmium's stability favoring physical studies and einsteinium's radioactivity supporting nuclear science investigations.
Availability and Sources
Osmium is one of the densest naturally occurring elements, primarily sourced from platinum ores found in South Africa and Russia, making it relatively accessible for research applications involving catalysis and material science. Einsteinium, a synthetic element produced in minute quantities through neutron bombardment in nuclear reactors or during nuclear explosions, faces extreme scarcity and high radioactivity, limiting its availability and usability in experimental research. The ease of obtaining osmium contrasts sharply with the complex, costly isolation and containment requirements of einsteinium, influencing their practical research deployment.
Handling and Safety Considerations
Osmium, a dense transition metal, requires careful handling due to its toxic osmium tetroxide compound, which can cause severe respiratory issues and skin irritation, necessitating well-ventilated workspaces and protective equipment. Einsteinium, a highly radioactive synthetic actinide, demands stringent radiological safety protocols, including glove boxes, remote handling tools, and specialized shielding to prevent exposure to harmful alpha and gamma radiation. Laboratories working with either element must implement rigorous monitoring and containment strategies to mitigate chemical toxicity and radiological hazards, ensuring researcher safety.
Cost and Rarity Comparison
Osmium, one of the densest naturally occurring elements, is significantly more affordable and accessible than einsteinium, a rare synthetic element produced in minute quantities in nuclear reactors. The high cost of einsteinium, driven by its scarcity and complex production process, limits its availability primarily to specialized nuclear research facilities. Osmium's relative abundance and lower price make it a more practical choice for extensive scientific research requiring dense metals, whereas einsteinium remains largely restricted to experimental studies in nuclear physics and radiochemistry.
Applications in Scientific Research
Osmium, a dense transition metal, is primarily utilized in catalysis, electron microscopy, and as a standard for mass measurement due to its high stability and hardness. Einsteinium, a synthetic actinide with no stable isotopes, serves mainly in nuclear research, particularly in studying transuranic elements and neutron capture processes. The contrasting applications highlight osmium's role in material science and microscopy, while einsteinium drives advancements in nuclear physics and elemental synthesis.
Chemical Reactivity and Stability
Osmium, a dense transition metal with atomic number 76, exhibits low chemical reactivity and exceptional stability, making it ideal for catalytic processes and corrosion-resistant applications in research. Einsteinium, a synthetic actinide element with atomic number 99, is highly radioactive and chemically reactive, limiting its experimental use to specialized nuclear and radiochemical studies. The stark contrast in chemical stability between osmium's inertness and einsteinium's radioactivity drives their distinct roles in scientific investigation.
Isotopes and Radioactivity
Osmium, a dense transition metal with stable isotopes like Osmium-192, exhibits low radioactivity making it suitable for precise scientific measurements and industrial applications. Einsteinium, a synthetic actinide element with no stable isotopes, is highly radioactive and primarily used in limited nuclear research and the study of heavy element synthesis. The stark contrast between Osmium's stable isotopes and Einsteinium's intense radioactivity defines their starkly different roles in advanced nuclear and chemical research.
Technological and Industrial Uses
Osmium's unparalleled density and hardness make it essential in industrial applications such as fountain pen nibs, electrical contacts, and catalytic converters, enabling advancements in precision engineering and electronics. Einsteinium, a synthetic, highly radioactive element with no stable isotopes, is primarily used in scientific research for synthesizing heavier transuranic elements and studying nuclear reactions, rather than direct technological or industrial applications. The contrasting properties underline osmium's practical utility in manufacturing and technology, while einsteinium's role remains confined to specialized nuclear research and element discovery.
Future Research Directions
Osmium's unparalleled density and catalytic properties position it as a critical material for advanced nanotechnology and sustainable energy research, driving innovations in fuel cell development and heterogeneous catalysis. Einsteinium, though limited by its scarcity and radioactivity, offers unique opportunities in nuclear science and the synthesis of superheavy elements, potentially expanding the periodic table and deepening understanding of atomic structure. Future research will likely explore osmium's applications in materials science and quantum computing, while einsteinium studies may focus on its role in neutron capture processes and advanced nuclear reactors.
Infographic: Osmium vs Einsteinium for Research
 azmater.com
azmater.com