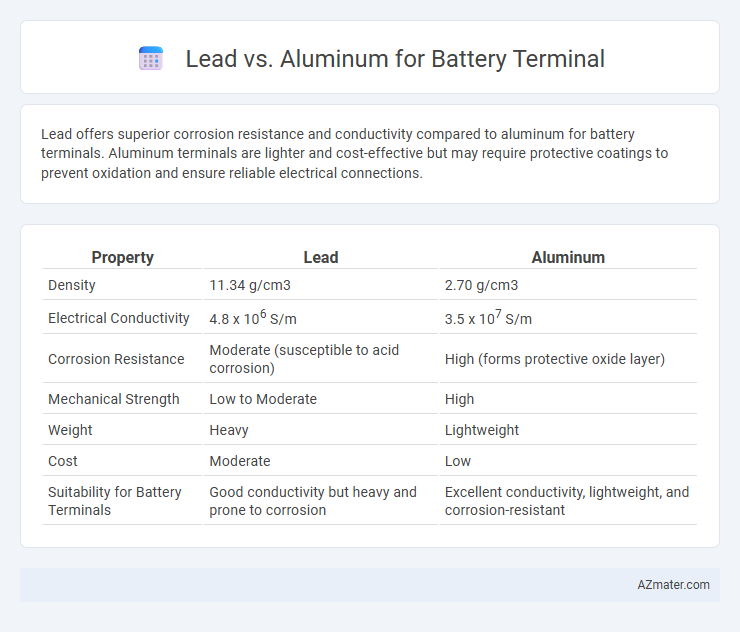
Lead offers superior corrosion resistance and conductivity compared to aluminum for battery terminals. Aluminum terminals are lighter and cost-effective but may require protective coatings to prevent oxidation and ensure reliable electrical connections.
Table of Comparison
| Property | Lead | Aluminum |
|---|---|---|
| Density | 11.34 g/cm3 | 2.70 g/cm3 |
| Electrical Conductivity | 4.8 x 106 S/m | 3.5 x 107 S/m |
| Corrosion Resistance | Moderate (susceptible to acid corrosion) | High (forms protective oxide layer) |
| Mechanical Strength | Low to Moderate | High |
| Weight | Heavy | Lightweight |
| Cost | Moderate | Low |
| Suitability for Battery Terminals | Good conductivity but heavy and prone to corrosion | Excellent conductivity, lightweight, and corrosion-resistant |
Introduction: Importance of Battery Terminals
Battery terminals are critical for ensuring efficient electrical conductivity and reliable power delivery in automotive and industrial applications. Lead terminals offer excellent corrosion resistance and durability, making them a traditional choice for heavy-duty batteries. Aluminum terminals provide a lightweight alternative with good conductivity but require protective coatings to prevent oxidation and maintain performance.
Overview of Lead and Aluminum Materials
Lead exhibits excellent corrosion resistance and superior conductivity, making it a traditional choice for battery terminals in automotive and industrial applications. Aluminum is lightweight, cost-effective, and offers good conductivity but is more prone to oxidation, which can affect terminal performance over time. The choice between lead and aluminum depends on factors such as durability requirements, cost constraints, and environmental considerations in battery terminal design.
Electrical Conductivity: Lead vs Aluminum
Aluminum offers higher electrical conductivity than lead, with approximately 61% of the conductivity of copper compared to lead's 7.2%, making aluminum more efficient for battery terminal applications requiring low resistance and high current flow. Despite lead's lower conductivity, its corrosion resistance and ease of welding often make it favorable in certain battery environments. Choosing between lead and aluminum depends on balancing electrical conductivity needs with mechanical properties and environmental factors.
Corrosion Resistance and Longevity
Lead battery terminals exhibit superior corrosion resistance due to their ability to form a stable oxide layer, reducing terminal degradation and ensuring reliable electrical connectivity over time. Aluminum terminals are more prone to oxidation and galvanic corrosion, which can lead to increased resistance and potential failure in battery performance. Consequently, lead terminals generally offer enhanced longevity and durability in demanding automotive and industrial battery applications.
Mechanical Strength and Durability
Lead offers superior mechanical strength and corrosion resistance for battery terminals, ensuring long-lasting durability under high-stress conditions. Aluminum, while lighter and more cost-effective, tends to have lower mechanical strength and is more prone to deformation and corrosion over time. Choosing lead results in enhanced terminal stability and reliability, especially in demanding automotive or industrial battery applications.
Cost Comparison and Economic Considerations
Lead battery terminals typically cost less upfront than aluminum, making them a more economical choice for standard automotive applications. Aluminum terminals, while often priced higher initially, offer long-term savings through reduced weight and improved corrosion resistance, which can lower maintenance expenses. Evaluating total cost of ownership, lead is cost-effective for short-term use, whereas aluminum presents financial benefits in performance-driven or lightweight vehicle designs.
Environmental Impact and Sustainability
Lead battery terminals pose significant environmental challenges due to toxic lead contamination and difficulties in recycling, which contribute to soil and water pollution. Aluminum terminals offer a more sustainable alternative with lower toxicity, higher recyclability, and reduced environmental footprint during extraction and processing. Choosing aluminum enhances battery sustainability by minimizing hazardous waste and supporting circular economy practices.
Compatibility with Battery Types
Lead battery terminals exhibit superior compatibility with lead-acid batteries due to their electrochemical stability and corrosion resistance, ensuring optimal conductivity and durability. Aluminum terminals, while lighter and more cost-effective, may cause galvanic corrosion when paired with lead-acid batteries unless properly coated or insulated. Compatibility concerns make lead terminals the preferred choice for traditional flooded, AGM, and gel lead-acid battery types.
Safety Considerations and Handling
Lead battery terminals offer superior chemical stability and corrosion resistance, reducing risks of acid exposure and electrical hazards during handling. Aluminum terminals require careful protection against oxidation, as aluminum oxide can increase resistance and potentially cause overheating or short circuits. Proper personal protective equipment (PPE) and maintenance protocols are essential when working with both metals to ensure safe installation and long-term battery performance.
Conclusion: Choosing the Right Battery Terminal Material
Lead battery terminals offer superior corrosion resistance and excellent conductivity, making them ideal for heavy-duty and long-lasting battery applications. Aluminum terminals provide a lightweight alternative with good conductivity but may require protective coatings to prevent oxidation and ensure durability. Selecting the right battery terminal material depends on balancing factors like weight, conductivity, corrosion resistance, and application-specific demands.
Infographic: Lead vs Aluminum for Battery Terminal
 azmater.com
azmater.com