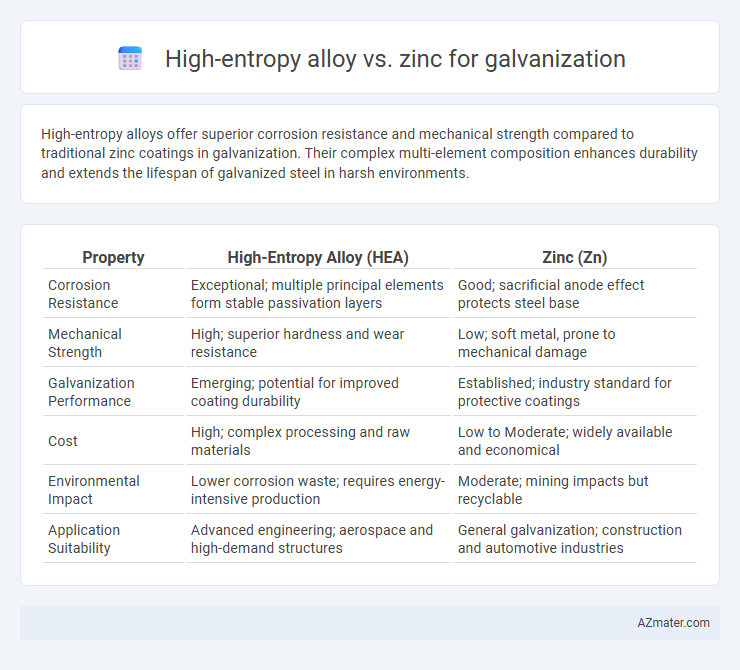
High-entropy alloys offer superior corrosion resistance and mechanical strength compared to traditional zinc coatings in galvanization. Their complex multi-element composition enhances durability and extends the lifespan of galvanized steel in harsh environments.
Table of Comparison
| Property | High-Entropy Alloy (HEA) | Zinc (Zn) |
|---|---|---|
| Corrosion Resistance | Exceptional; multiple principal elements form stable passivation layers | Good; sacrificial anode effect protects steel base |
| Mechanical Strength | High; superior hardness and wear resistance | Low; soft metal, prone to mechanical damage |
| Galvanization Performance | Emerging; potential for improved coating durability | Established; industry standard for protective coatings |
| Cost | High; complex processing and raw materials | Low to Moderate; widely available and economical |
| Environmental Impact | Lower corrosion waste; requires energy-intensive production | Moderate; mining impacts but recyclable |
| Application Suitability | Advanced engineering; aerospace and high-demand structures | General galvanization; construction and automotive industries |
Introduction to Galvanization: Purpose and Methods
Galvanization involves applying a protective zinc coating to steel or iron to prevent rusting, commonly through hot-dip or electro-galvanizing techniques. High-entropy alloys (HEAs) offer a novel alternative with superior corrosion resistance due to their complex, multi-element compositions, which can enhance longevity compared to traditional zinc coatings. Understanding the differing properties and application methods of zinc and HEAs is crucial for advancing galvanization technologies in industrial corrosion protection.
Overview of High-Entropy Alloys
High-entropy alloys (HEAs) are composed of multiple principal elements in near-equiatomic ratios, offering exceptional mechanical strength, corrosion resistance, and thermal stability compared to traditional zinc coatings used in galvanization. Unlike zinc, which primarily serves as a sacrificial anode to protect steel from rust through galvanic corrosion, HEAs provide a more durable and wear-resistant protective layer that can endure harsh environmental conditions. Research indicates that HEA coatings can enhance the service life of galvanized materials while reducing maintenance and replacement costs.
Zinc: Traditional Choice for Galvanization
Zinc remains the traditional and widely used choice for galvanization due to its excellent corrosion resistance and cost-effectiveness. It forms a protective oxide layer that prevents further metal oxidation, extending the lifespan of steel structures. High-entropy alloys, while promising for advanced applications, currently lack the established infrastructure and economic viability that zinc provides in large-scale galvanization processes.
Corrosion Resistance: High-Entropy Alloy vs. Zinc
High-entropy alloys (HEAs) exhibit superior corrosion resistance compared to traditional zinc coatings due to their complex multi-element composition, which enhances passivation and reduces localized corrosion. Zinc galvanization provides effective sacrificial protection by preferentially corroding, but it is more susceptible to degradation in aggressive environments such as high chloride or acidic conditions. Advanced HEAs demonstrate improved long-term durability and resistance to pitting and crevice corrosion, making them an emerging alternative for protective coatings in harsh industrial applications.
Mechanical Properties Comparison
High-entropy alloys (HEAs) demonstrate superior mechanical properties compared to zinc in galvanization, offering enhanced strength, hardness, and wear resistance due to their multi-element composition and unique microstructure. Zinc coatings provide moderate corrosion protection but have lower tensile strength and ductility, limiting mechanical durability under stress. HEAs' exceptional fracture toughness and resistance to deformation make them promising alternatives for galvanized layers in demanding industrial applications.
Environmental Impact and Sustainability
High-entropy alloys (HEAs) offer enhanced corrosion resistance and longer lifespan compared to traditional zinc coatings, reducing frequent reapplications and associated waste. Zinc galvanization, while cost-effective and widely used, poses environmental concerns such as heavy metal runoff and limited recyclability. The sustainability of HEAs stems from their durability and potential for eco-friendly alloying elements, making them a promising alternative to zinc in galvanization processes.
Cost Analysis and Economic Viability
High-entropy alloys (HEAs) offer superior corrosion resistance compared to traditional zinc coatings, potentially reducing maintenance costs over time, but their high production cost currently limits widespread economic viability. Zinc galvanization remains the preferred choice due to its low cost, abundant availability, and well-established industrial processes, making it economically favorable for large-scale applications. Cost analysis reveals zinc's affordability and scalability outweigh HEAs' performance benefits in most commercial galvanization scenarios.
Lifespan and Durability in Real-world Applications
High-entropy alloys (HEAs) exhibit superior corrosion resistance and mechanical strength compared to traditional zinc coatings, significantly extending the lifespan of galvanized materials in harsh environments. Zinc offers effective sacrificial protection but tends to degrade faster under prolonged exposure to moisture and chemicals, limiting its durability in real-world applications. Real-world data shows HEA-coated surfaces maintain structural integrity and resist wear longer, reducing maintenance frequency and overall lifecycle costs in industrial and infrastructure projects.
Industrial Adoption and Future Prospects
High-entropy alloys (HEAs) present a revolutionary alternative to traditional zinc coatings in galvanization, offering superior corrosion resistance and mechanical strength that suit heavy industrial applications. While zinc remains dominant due to cost-effectiveness and established supply chains, HEAs are gaining traction in sectors such as aerospace and automotive, where performance outweighs material cost. Emerging research on scalable production methods and alloy customization signals promising industrial adoption and long-term market potential for HEAs in advanced galvanization.
Conclusion: Choosing the Optimal Galvanization Material
High-entropy alloys (HEAs) offer superior corrosion resistance and mechanical strength compared to traditional zinc coatings, making them suitable for extreme environments where durability is critical. Zinc remains cost-effective and widely used for galvanization due to its excellent sacrificial protection and ease of application on steel surfaces. Selecting between HEAs and zinc depends on balancing factors such as environmental conditions, budget constraints, and long-term performance requirements.
Infographic: High-entropy alloy vs Zinc for Galvanization
 azmater.com
azmater.com