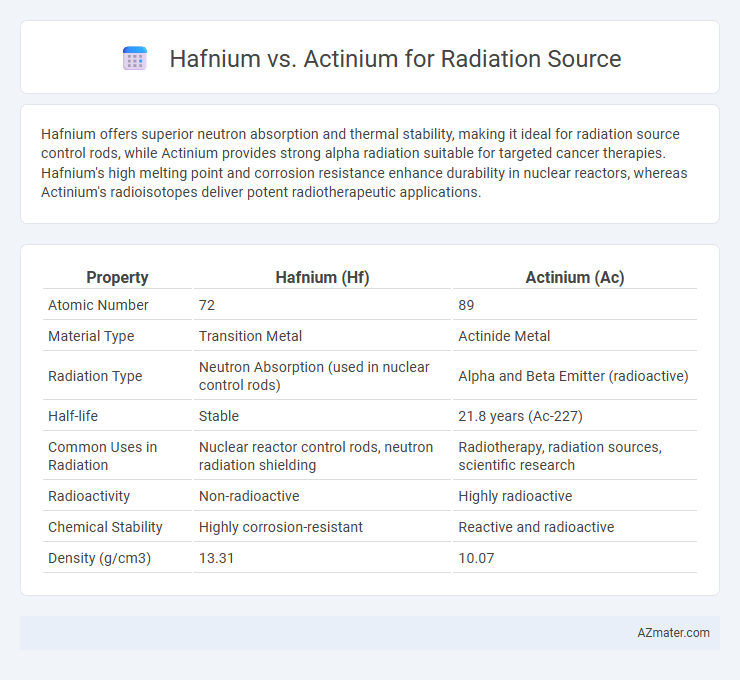
Hafnium offers superior neutron absorption and thermal stability, making it ideal for radiation source control rods, while Actinium provides strong alpha radiation suitable for targeted cancer therapies. Hafnium's high melting point and corrosion resistance enhance durability in nuclear reactors, whereas Actinium's radioisotopes deliver potent radiotherapeutic applications.
Table of Comparison
| Property | Hafnium (Hf) | Actinium (Ac) |
|---|---|---|
| Atomic Number | 72 | 89 |
| Material Type | Transition Metal | Actinide Metal |
| Radiation Type | Neutron Absorption (used in nuclear control rods) | Alpha and Beta Emitter (radioactive) |
| Half-life | Stable | 21.8 years (Ac-227) |
| Common Uses in Radiation | Nuclear reactor control rods, neutron radiation shielding | Radiotherapy, radiation sources, scientific research |
| Radioactivity | Non-radioactive | Highly radioactive |
| Chemical Stability | Highly corrosion-resistant | Reactive and radioactive |
| Density (g/cm3) | 13.31 | 10.07 |
Introduction to Hafnium and Actinium as Radiation Sources
Hafnium and actinium are both utilized as radiation sources in various applications, with hafnium known for its neutron absorption properties in nuclear reactors due to its high neutron capture cross-section. Actinium, a radioactive actinide, emits alpha particles and is employed in targeted radiation therapy and industrial radiography. The distinct nuclear characteristics of hafnium and actinium determine their suitability for specific radiation-based technologies and medical uses.
Atomic Structure and Isotopic Properties
Hafnium, with an atomic number of 72, possesses a stable electron configuration [Xe] 4f14 5d2 6s2, contributing to its chemical stability and suitability in neutron capture applications for radiation sources. Actinium, atomic number 89, exhibits a highly radioactive nature due to its position in the actinide series and has multiple isotopes, notably Ac-227, with significant alpha emission used in targeted radiation therapies. The isotopic properties of actinium, including its half-life of 21.77 years for Ac-227, contrast with the stable isotopes of hafnium, making actinium more effective for high-intensity radiation sources, whereas hafnium's neutron absorption cross-section is leveraged in nuclear reactor control.
Radiological Characteristics and Emission Types
Hafnium, known for its high neutron absorption cross-section, primarily emits gamma radiation and is widely used in control rods for nuclear reactors, offering high radiological stability and effective neutron capture. Actinium, a naturally radioactive element, predominantly emits alpha particles along with beta and gamma radiation, exhibiting intense radioactivity and a relatively short half-life, making it suitable for targeted alpha therapy and radiation sources in medical applications. The differing emission types and radiological characteristics of hafnium and actinium determine their distinct roles in radiation source technology, with hafnium favored for neutron control and actinium valued for its potent alpha emissions.
Availability and Natural Occurrence
Hafnium is a relatively abundant element in the Earth's crust, typically found in zirconium minerals with a concentration of about 5.8 parts per million, making it more accessible for use as a radiation source. Actinium, on the other hand, is extremely rare and occurs naturally in trace amounts within uranium and thorium ores, often less than 1 part per million, complicating its extraction and availability. The scarcity of actinium results in higher production costs and limited supply compared to hafnium, influencing their practical applications in radiation technologies.
Efficiency as Radiation Sources
Hafnium exhibits exceptional neutron absorption efficiency due to its high neutron capture cross-section, making it highly effective in nuclear reactors and radiation shielding applications. Actinium, while radioactive, primarily emits alpha particles and has lower neutron interaction efficiency compared to hafnium, limiting its use as a neutron radiation source. The superior neutron capture properties of hafnium enable more efficient radiation control and energy absorption in nuclear systems.
Safety and Handling Protocols
Hafnium, with its high neutron absorption cross-section, is favored in radiation sources due to relatively stable chemical properties and lower radioactivity, enabling safer handling protocols compared to Actinium, which is highly radioactive and requires stringent containment measures to prevent alpha particle exposure. Actinium's intense radioactivity demands specialized equipment, remote handling, and rigorous shielding to protect against radiological hazards, while Hafnium's metallic form allows for standard industrial safety practices. Proper personal protective equipment (PPE) and adherence to regulatory guidelines are crucial for both elements, but the increased radiotoxicity of Actinium necessitates more comprehensive radiation monitoring and waste disposal procedures.
Applications in Medical and Industrial Fields
Hafnium is primarily used in medical radiation therapy devices due to its high neutron absorption and stability, making it ideal for cancer treatment and radiation shielding. Actinium, particularly Actinium-225, is valuable in targeted alpha therapy for cancer, offering potent cell-killing capabilities with minimal damage to surrounding tissues. Industrial applications favor hafnium for neutron control in nuclear reactors, while actinium's radioactive properties are leveraged for scientific research and specialized radiopharmaceuticals.
Environmental Impact and Disposal
Hafnium, with its stable isotopes and relatively low radioactivity, presents fewer environmental risks compared to Actinium, which is highly radioactive and poses significant contamination hazards during disposal. The long half-life and intense alpha radiation of Actinium demand stringent containment to prevent soil and water pollution, complicating waste management and increasing environmental impact. Hafnium's chemical inertness and lower radiotoxicity facilitate safer disposal processes, reducing long-term ecological damage and making it a more environmentally favorable option for radiation sources.
Regulatory Considerations
Hafnium and Actinium are both used as radiation sources, but regulatory considerations differ due to their distinct radiological properties and applications. Hafnium, often utilized in control rods and neutron absorption, is subject to regulations focused on nuclear safety and criticality control under agencies like the NRC and IAEA. Actinium, primarily used in targeted alpha therapy and industrial radiography, faces stricter handling and disposal regulations due to its higher radioactivity and longer half-life, necessitating compliance with guidelines from bodies such as the EPA and OSHA.
Comparative Summary: Hafnium vs Actinium
Hafnium and Actinium differ significantly as radiation sources due to their distinct nuclear properties and applications. Hafnium, known for its high neutron absorption cross-section, is valuable in control rods for nuclear reactors, offering precise neutron regulation and stability under high temperatures. Actinium, a rare radioactive element with strong alpha radiation, serves primarily in targeted alpha therapy for cancer treatment, providing potent localized radiation but with challenges in handling and availability.
Infographic: Hafnium vs Actinium for Radiation Source
 azmater.com
azmater.com