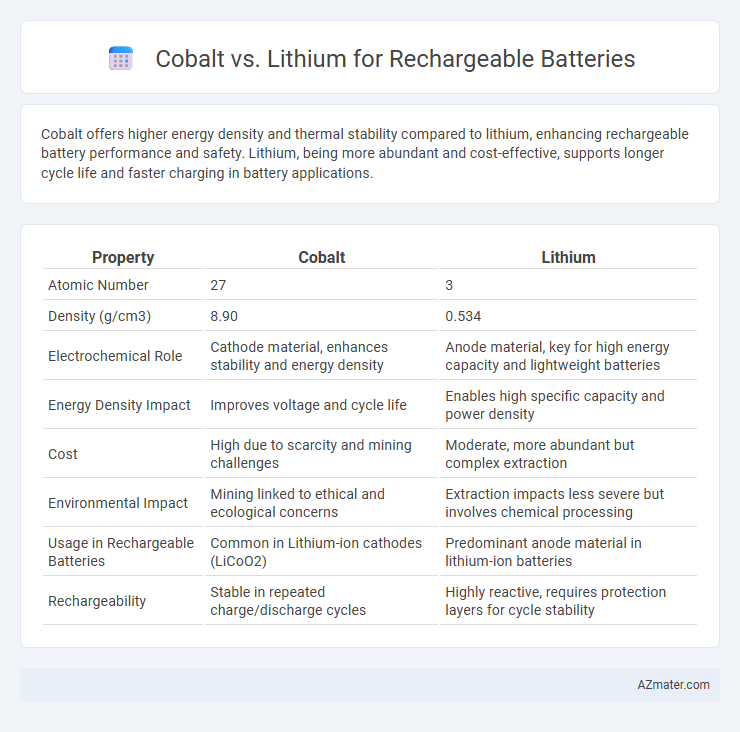
Cobalt offers higher energy density and thermal stability compared to lithium, enhancing rechargeable battery performance and safety. Lithium, being more abundant and cost-effective, supports longer cycle life and faster charging in battery applications.
Table of Comparison
| Property | Cobalt | Lithium |
|---|---|---|
| Atomic Number | 27 | 3 |
| Density (g/cm3) | 8.90 | 0.534 |
| Electrochemical Role | Cathode material, enhances stability and energy density | Anode material, key for high energy capacity and lightweight batteries |
| Energy Density Impact | Improves voltage and cycle life | Enables high specific capacity and power density |
| Cost | High due to scarcity and mining challenges | Moderate, more abundant but complex extraction |
| Environmental Impact | Mining linked to ethical and ecological concerns | Extraction impacts less severe but involves chemical processing |
| Usage in Rechargeable Batteries | Common in Lithium-ion cathodes (LiCoO2) | Predominant anode material in lithium-ion batteries |
| Rechargeability | Stable in repeated charge/discharge cycles | Highly reactive, requires protection layers for cycle stability |
Introduction to Cobalt and Lithium in Rechargeable Batteries
Cobalt and lithium are essential materials in rechargeable battery technology, each playing a distinct role in battery performance and safety. Lithium, used primarily as lithium-ion, acts as the primary charge carrier enabling high energy density and efficient charge-discharge cycles, while cobalt enhances battery stability, energy density, and longevity by stabilizing the cathode structure. The balance between lithium's energy capacity and cobalt's structural support is critical for optimizing rechargeable battery efficiency and durability in applications such as electric vehicles and portable electronics.
Chemical Properties: Cobalt vs Lithium
Cobalt exhibits a high electrochemical stability and excellent capacity retention, making it essential for stabilizing lithium-ion battery cathodes, especially in layered oxide materials like LiCoO2. Lithium, characterized by its low atomic mass and high electrochemical potential, is key for energy density and battery voltage, serving as the primary charge carrier in rechargeable batteries. The chemical properties of cobalt enhance thermal stability and cycle life, while lithium's reactivity and lightness drive energy storage efficiency.
Energy Density Comparison
Cobalt and lithium play distinct roles in the energy density of rechargeable batteries, with lithium-ion batteries typically delivering higher energy density due to lithium's lighter atomic weight and electrochemical properties. Cobalt is primarily used in cathode materials such as lithium cobalt oxide (LiCoO2), enhancing battery stability and longevity but adding weight and cost, which can slightly reduce overall energy density compared to cobalt-free chemistries. Advances in lithium-based cathodes, including nickel-cobalt-manganese (NCM) and lithium iron phosphate (LFP) batteries, aim to optimize energy density by balancing cobalt content with lithium's high capacity potential.
Cost Analysis: Cobalt versus Lithium Batteries
Cobalt batteries typically incur higher production costs due to the metal's scarcity and ethical sourcing issues, driving prices above those of lithium-only alternatives. Lithium batteries, especially lithium iron phosphate (LFP) types, offer a cost-effective solution with more stable pricing and abundant raw materials, reducing overall battery expenses. Manufacturers increasingly favor lithium-based chemistries to balance performance and affordability in large-scale rechargeable battery production.
Performance and Efficiency Differences
Cobalt-based cathodes exhibit higher energy density and better thermal stability, enhancing battery performance under high-load conditions, while lithium metal anodes contribute to faster charging rates and longer cycle life. Lithium-ion batteries with reduced cobalt content improve energy efficiency and reduce overall weight, optimizing electric vehicle range and battery lifespan. Balancing cobalt's stability with lithium's conductivity remains crucial for advancing rechargeable battery technology in terms of performance and efficiency.
Safety Considerations for Cobalt and Lithium Batteries
Cobalt-based batteries, commonly found in lithium-ion chemistries, provide high energy density but pose safety risks due to thermal runaway and potential toxic cobalt exposure during overheating or damage. Lithium batteries, especially lithium iron phosphate (LiFePO4), offer enhanced thermal stability and reduced fire hazards, making them a safer alternative for applications requiring high safety standards. Proper battery management systems and cell design significantly mitigate risks in both cobalt and lithium chemistries, ensuring safer operation in consumer electronics and electric vehicles.
Environmental Impact and Sustainability
Cobalt mining generates significant environmental concerns due to toxic waste and habitat destruction, while lithium extraction primarily impacts water resources and local ecosystems. Recycling rates for lithium-ion batteries remain low, limiting the reduction of raw material demand and environmental footprint for both metals. Sustainable alternatives and improved recycling technologies are critical to mitigating the ecological damage associated with cobalt and lithium in rechargeable batteries.
Resource Availability and Mining Concerns
Cobalt reserves are geographically concentrated, primarily in the Democratic Republic of Congo, raising significant geopolitical and ethical mining concerns, including child labor and environmental degradation. Lithium is more widely distributed, with abundant deposits in Australia, Chile, and Argentina, yet its extraction involves substantial water consumption and ecosystem disruption in arid regions. The contrasting resource availability and mining impacts make cobalt less sustainable and lithium a relatively more accessible but environmentally challenging option for rechargeable battery production.
Current Applications and Industry Trends
Cobalt remains a critical component in lithium-ion batteries due to its role in stabilizing cathodes and enhancing energy density, with significant usage in electric vehicle batteries and consumer electronics. Recent industry trends emphasize reducing cobalt content to address ethical concerns and supply chain risks, leading to the rise of lithium iron phosphate (LFP) and nickel-rich battery chemistries that either minimize or eliminate cobalt. The shift reflects a balance between maintaining battery performance, cost-effectiveness, and sustainable sourcing in large-scale energy storage and automotive applications.
Future Prospects: Innovations in Battery Technology
Cobalt and lithium remain critical materials in rechargeable battery technology, with ongoing innovations aiming to reduce cobalt dependency due to its cost and ethical concerns. Emerging solid-state batteries and lithium-sulfur chemistries promise higher energy densities and improved safety, potentially minimizing cobalt usage while enhancing performance. Advanced recycling techniques and alternative cathode materials like nickel-rich and manganese-based compounds are driving sustainable development in next-generation batteries for electric vehicles and renewable energy storage.
Infographic: Cobalt vs Lithium for Rechargeable Battery
 azmater.com
azmater.com