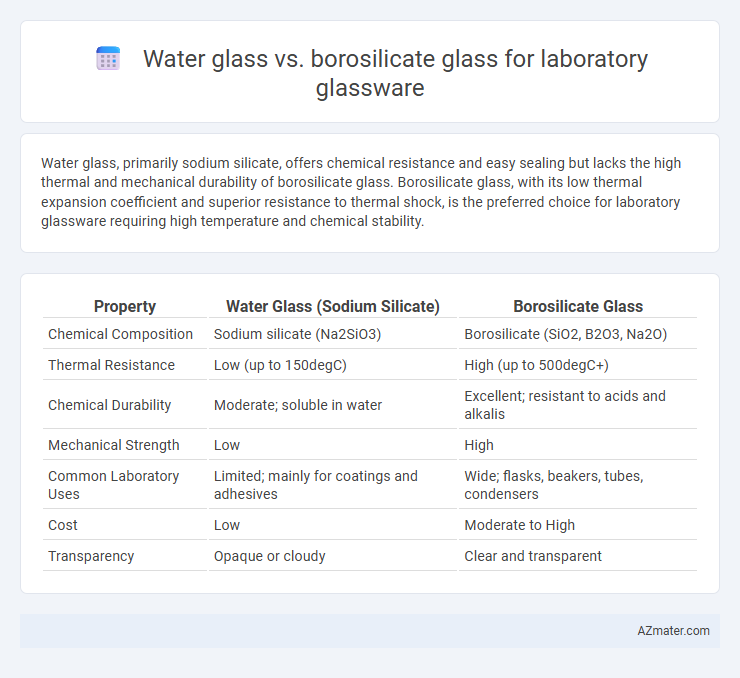
Water glass, primarily sodium silicate, offers chemical resistance and easy sealing but lacks the high thermal and mechanical durability of borosilicate glass. Borosilicate glass, with its low thermal expansion coefficient and superior resistance to thermal shock, is the preferred choice for laboratory glassware requiring high temperature and chemical stability.
Table of Comparison
| Property | Water Glass (Sodium Silicate) | Borosilicate Glass |
|---|---|---|
| Chemical Composition | Sodium silicate (Na2SiO3) | Borosilicate (SiO2, B2O3, Na2O) |
| Thermal Resistance | Low (up to 150degC) | High (up to 500degC+) |
| Chemical Durability | Moderate; soluble in water | Excellent; resistant to acids and alkalis |
| Mechanical Strength | Low | High |
| Common Laboratory Uses | Limited; mainly for coatings and adhesives | Wide; flasks, beakers, tubes, condensers |
| Cost | Low | Moderate to High |
| Transparency | Opaque or cloudy | Clear and transparent |
Introduction to Laboratory Glassware Materials
Water glass, or sodium silicate, is primarily used as a sealing and adhesive agent in laboratory contexts but is not suitable for direct use as glassware material due to its lower thermal and chemical resistance. Borosilicate glass, composed mainly of silica and boron trioxide, is the preferred material for laboratory glassware because of its outstanding thermal shock resistance, low thermal expansion coefficient (approximately 3.3 x 10^-6 /degC), and excellent chemical durability against acids, bases, and solvents. These properties make borosilicate glass the industry standard for high-precision lab applications such as beakers, flasks, and pipettes.
What is Water Glass?
Water glass, also known as sodium silicate, is a liquid solution primarily composed of sodium oxide and silica used in laboratories for sealing and preservative applications rather than making glassware. Unlike borosilicate glass, which is a solid material known for its thermal resistance and chemical durability in laboratory glassware, water glass functions as a bonding and protective agent. Its unique properties make it suitable for tasks such as preserving eggs or fixing stains, but it does not provide the mechanical strength required for reusable laboratory containers.
What is Borosilicate Glass?
Borosilicate glass is a type of laboratory glassware known for its exceptional thermal resistance and chemical durability, composed primarily of silica and boron trioxide. Unlike water glass, it withstands rapid temperature changes without cracking, making it ideal for experiments involving heat. Its low coefficient of thermal expansion and high resistance to chemical corrosion ensure precise and safe laboratory applications.
Chemical Composition Comparison
Water glass, composed primarily of sodium silicate (Na2SiO3), differs significantly from borosilicate glass, which contains silica (SiO2) and boron trioxide (B2O3) as its main components. Borosilicate glass offers superior chemical resistance and thermal stability due to its low alkali content and the presence of boron oxide, making it ideal for laboratory glassware exposed to acidic and high-temperature environments. In contrast, water glass's higher sodium oxide content results in lower durability and chemical resistance, limiting its use in laboratory applications.
Thermal Resistance: Water Glass vs Borosilicate
Borosilicate glass offers superior thermal resistance compared to water glass, tolerating temperatures up to 450degC without deformation or cracking, which is essential for rigorous laboratory heating processes. Water glass, primarily a sodium silicate solution, lacks solid form stability and is not suitable for direct thermal applications in lab glassware. The enhanced thermal resistance of borosilicate glass ensures durability and safety during repeated thermal cycling in scientific experiments.
Mechanical Strength and Durability
Borosilicate glass exhibits superior mechanical strength and thermal shock resistance compared to water glass, making it ideal for laboratory glassware subjected to rapid temperature changes and mechanical stress. Water glass, primarily sodium silicate solutions, lacks the structural integrity of borosilicate, reducing its durability under laboratory conditions. The enhanced durability of borosilicate is due to its low coefficient of thermal expansion, which minimizes cracking and extends the lifespan of lab equipment.
Chemical Resistance in Laboratory Environments
Borosilicate glass exhibits superior chemical resistance compared to water glass, making it ideal for laboratory glassware exposed to strong acids, bases, and organic solvents. Its low thermal expansion coefficient also enhances durability against rapid temperature changes, reducing the risk of breakage during experiments. Water glass, primarily used in construction, lacks the chemical stability required for harsh laboratory environments and is generally unsuitable for precise scientific applications.
Cost and Availability
Water glass, also known as sodium silicate glass, is generally more affordable and widely available due to its simple composition and mass production, making it a cost-effective option for basic laboratory glassware. Borosilicate glass, prized for its superior thermal resistance and chemical durability, tends to be more expensive and may have limited availability depending on suppliers specializing in high-quality lab equipment. Laboratories requiring precise heat treatment or chemical resistance often invest in borosilicate glass despite its higher cost to ensure reliability and safety.
Suitability for Laboratory Applications
Borosilicate glass is highly suitable for laboratory applications due to its excellent thermal resistance, chemical durability, and low thermal expansion, making it ideal for heating and cooling processes. Water glass, or sodium silicate, lacks these thermal and chemical stability properties, rendering it less appropriate for precise laboratory environments. Therefore, borosilicate glass remains the preferred choice for laboratory glassware requiring durability and resistance to thermal shock.
Conclusion: Choosing the Right Glassware
For laboratory glassware, borosilicate glass offers superior thermal resistance and chemical durability compared to water glass, making it ideal for high-temperature and corrosive experiments. Water glass, while cost-effective and suitable for basic uses, lacks the mechanical strength and thermal stability required in advanced laboratory settings. Selecting borosilicate glass enhances safety, longevity, and reliability in scientific applications that demand precise and consistent performance.
Infographic: Water glass vs Borosilicate glass for Laboratory glassware
 azmater.com
azmater.com