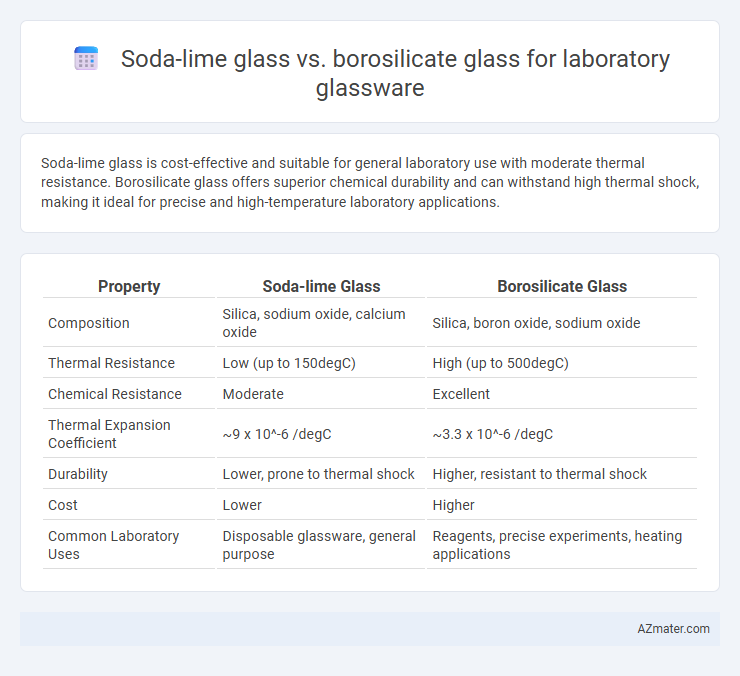
Soda-lime glass is cost-effective and suitable for general laboratory use with moderate thermal resistance. Borosilicate glass offers superior chemical durability and can withstand high thermal shock, making it ideal for precise and high-temperature laboratory applications.
Table of Comparison
| Property | Soda-lime Glass | Borosilicate Glass |
|---|---|---|
| Composition | Silica, sodium oxide, calcium oxide | Silica, boron oxide, sodium oxide |
| Thermal Resistance | Low (up to 150degC) | High (up to 500degC) |
| Chemical Resistance | Moderate | Excellent |
| Thermal Expansion Coefficient | ~9 x 10^-6 /degC | ~3.3 x 10^-6 /degC |
| Durability | Lower, prone to thermal shock | Higher, resistant to thermal shock |
| Cost | Lower | Higher |
| Common Laboratory Uses | Disposable glassware, general purpose | Reagents, precise experiments, heating applications |
Introduction to Laboratory Glassware Materials
Soda-lime glass, composed primarily of silica, sodium oxide, and calcium oxide, offers cost-effective and durable options for general laboratory glassware but has limited thermal resistance and chemical durability compared to borosilicate glass. Borosilicate glass contains silica and boron trioxide, providing superior thermal shock resistance and chemical stability, making it ideal for applications involving high temperatures and corrosive substances. Laboratories often prefer borosilicate glassware for precise experiments due to its reliability under extreme conditions, while soda-lime glass is commonly used for less demanding tasks.
Composition of Soda-Lime Glass
Soda-lime glass, primarily composed of about 70-75% silica (SiO2), 12-15% soda (Na2O), and 5-10% lime (CaO), offers affordability and ease of manufacturing for laboratory glassware. The high soda content lowers the melting temperature but reduces chemical durability and thermal resistance compared to borosilicate glass, which contains boron oxide (B2O3) for enhanced thermal shock resistance. This compositional difference makes soda-lime glass less suitable for applications requiring exposure to rapid temperature changes or aggressive chemicals.
Composition of Borosilicate Glass
Borosilicate glass, commonly used in laboratory glassware, consists primarily of silica (SiO2) around 70-80% and boron oxide (B2O3) about 7-13%, which imparts enhanced thermal and chemical resistance compared to soda-lime glass. The presence of boron oxide reduces thermal expansion, making borosilicate glass highly resistant to thermal shock and ideal for precise scientific experiments. This composition contrasts with soda-lime glass that contains higher amounts of sodium oxide (Na2O) and calcium oxide (CaO), offering less durability under rapid temperature changes and chemical exposure.
Thermal Resistance and Stability
Borosilicate glass offers superior thermal resistance with a softening point around 820degC, making it highly stable for laboratory applications involving rapid temperature changes or high heat exposure. Soda-lime glass, with a lower softening point near 600degC, is less resistant to thermal shock and more prone to cracking under sudden temperature fluctuations. The enhanced thermal stability of borosilicate glass ensures reliable performance in precise scientific experiments requiring consistent chemical and physical properties.
Chemical Durability and Corrosion Resistance
Soda-lime glass, composed primarily of sodium oxide, calcium oxide, and silica, offers moderate chemical durability but is prone to corrosion from alkalis and hydrofluoric acid, making it less suitable for aggressive chemical environments. Borosilicate glass contains boron trioxide, enhancing its chemical resistance and thermal stability, which provides superior corrosion resistance against acids, bases, and organic solvents commonly used in laboratories. This enhanced durability makes borosilicate glass the preferred choice for laboratory glassware requiring high resistance to chemical attack and thermal shock.
Mechanical Strength and Durability
Borosilicate glass exhibits superior mechanical strength and durability compared to soda-lime glass, making it highly resistant to thermal shock and mechanical stress in laboratory environments. Soda-lime glass is more prone to cracking or breaking under rapid temperature changes due to its lower thermal resistance and weaker structural integrity. Laboratories favor borosilicate glassware for applications requiring repeated heating or exposure to harsh chemicals, ensuring longer service life and reduced risk of breakage.
Cost and Availability Comparison
Soda-lime glass is widely preferred in laboratory glassware due to its lower cost and higher availability compared to borosilicate glass, making it an economical choice for general use. Borosilicate glass, although more expensive, offers superior thermal resistance and chemical durability, which justifies its price in specialized applications requiring high precision and heat resistance. The cost difference arises from the raw materials and manufacturing process, where borosilicate's unique composition demands more complex production, impacting its market availability and pricing.
Common Laboratory Applications
Soda-lime glass is widely used for general-purpose laboratory glassware such as beakers, test tubes, and bottles due to its affordability and good chemical resistance for neutral solutions. Borosilicate glass is preferred for applications requiring high thermal resistance and durability, making it ideal for heating, autoclaving, and handling reactive chemicals in lab settings. Common laboratory uses of borosilicate include volumetric flasks, condensers, and pipettes, which demand superior thermal shock resistance and mechanical strength.
Safety Considerations and Breakage Risks
Soda-lime glass, commonly used in laboratory glassware, has lower resistance to thermal shock and chemical corrosion, increasing the risk of breakage during rapid temperature changes or exposure to aggressive chemicals. Borosilicate glass offers superior safety due to its high thermal resistance and chemical durability, significantly reducing the likelihood of cracks and shattering under extreme lab conditions. Choosing borosilicate glass minimizes safety hazards and enhances the lifespan of laboratory equipment in demanding research environments.
Choosing the Right Glassware for Your Laboratory
Soda-lime glass is cost-effective and suitable for general laboratory applications requiring chemical resistance to dilute acids and alkalis but has lower thermal resistance and is prone to thermal shock. Borosilicate glass offers superior thermal and chemical resistance, making it ideal for experiments involving rapid temperature changes or harsh chemicals. Choosing the right glassware depends on the specific laboratory needs, balancing cost and performance, with borosilicate preferred for durability and thermal stability.
Infographic: Soda-lime glass vs Borosilicate glass for Laboratory glassware
 azmater.com
azmater.com