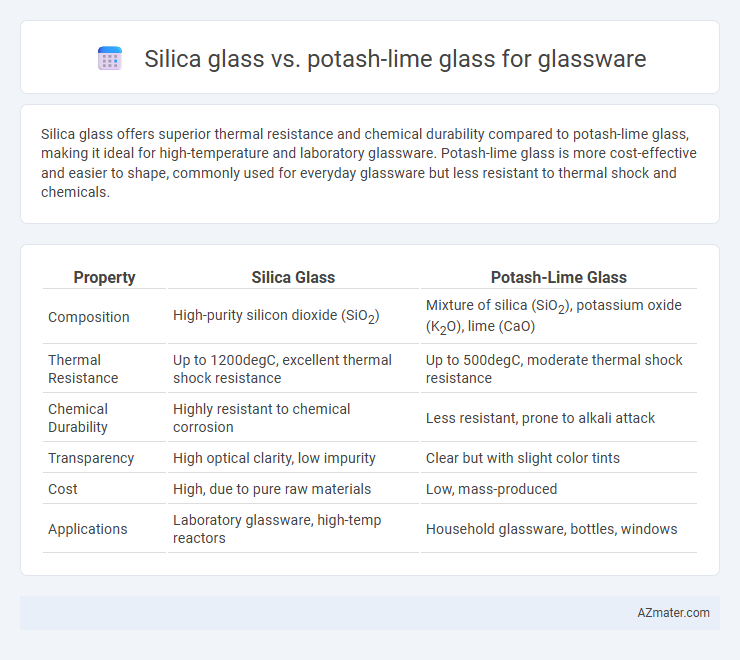
Silica glass offers superior thermal resistance and chemical durability compared to potash-lime glass, making it ideal for high-temperature and laboratory glassware. Potash-lime glass is more cost-effective and easier to shape, commonly used for everyday glassware but less resistant to thermal shock and chemicals.
Table of Comparison
| Property | Silica Glass | Potash-Lime Glass |
|---|---|---|
| Composition | High-purity silicon dioxide (SiO2) | Mixture of silica (SiO2), potassium oxide (K2O), lime (CaO) |
| Thermal Resistance | Up to 1200degC, excellent thermal shock resistance | Up to 500degC, moderate thermal shock resistance |
| Chemical Durability | Highly resistant to chemical corrosion | Less resistant, prone to alkali attack |
| Transparency | High optical clarity, low impurity | Clear but with slight color tints |
| Cost | High, due to pure raw materials | Low, mass-produced |
| Applications | Laboratory glassware, high-temp reactors | Household glassware, bottles, windows |
Introduction to Glass Types Used in Glassware
Silica glass, primarily composed of silicon dioxide, offers superior thermal resistance and chemical durability compared to potash-lime glass, which contains potassium oxide and calcium oxide as major components. Potash-lime glass is commonly used for everyday glassware due to its cost-effectiveness and ease of shaping, while silica glass is preferred in high-performance applications requiring higher purity and durability. The choice between silica and potash-lime glass depends on factors such as thermal stability, chemical resistance, and manufacturing costs.
Composition Differences: Silica Glass vs Potash-Lime Glass
Silica glass primarily consists of silicon dioxide (SiO2) with minimal additives, resulting in high purity and superior thermal and chemical resistance. Potash-lime glass contains a mixture of silica, potassium oxide (K2O), and lime (CaO), which lowers melting temperature and production costs but reduces durability and heat resistance compared to silica glass. These compositional differences significantly impact the performance and application of glassware, with silica glass preferred for laboratory and high-temperature uses, while potash-lime glass is common in everyday containers and decorative items.
Manufacturing Processes Compared
Silica glass, primarily composed of silicon dioxide (SiO2), is manufactured through high-temperature melting of pure quartz sand, requiring temperatures above 1700degC, resulting in exceptional thermal and chemical resistance for premium glassware. Potash-lime glass, produced by melting silica with potassium carbonate and lime at lower temperatures around 1400degC, offers cost-effective production but lower durability and thermal stability compared to silica glass. The manufacturing process of silica glass demands precise control and longer annealing times, enhancing structural uniformity, while potash-lime glass benefits from faster cooling and ease of shaping, making it prevalent in everyday glassware.
Optical Clarity and Transparency
Silica glass offers superior optical clarity and higher transparency compared to potash-lime glass, making it ideal for high-precision glassware applications. The low iron content in silica glass reduces color tints and enhances light transmission, resulting in exceptionally clear and colorless glass. Potash-lime glass, while more economical, typically exhibits lower transparency and may have slight greenish or brownish hues due to its higher metal oxide content.
Chemical Durability and Resistance
Silica glass exhibits superior chemical durability and resistance compared to potash-lime glass, making it highly effective against acidic and alkaline substances. Its high purity and strong silicon-oxygen bonds enhance resistance to corrosion and leaching, ensuring long-term stability in laboratory and industrial applications. Potash-lime glass, while more affordable, is more susceptible to chemical attack and degradation over time, limiting its use in environments with aggressive chemicals.
Thermal Properties and Heat Tolerance
Silica glass exhibits superior thermal stability with a high softening point around 1700degC, making it ideal for demanding high-temperature applications and resistance to thermal shock. Potash-lime glass, with a lower softening point near 700degC, has limited heat tolerance and is more prone to deformation under rapid temperature changes. The low thermal expansion coefficient of silica glass significantly reduces stress fractures compared to potash-lime glass in glassware exposed to fluctuating temperatures.
Mechanical Strength and Fragility
Silica glass exhibits superior mechanical strength and resistance to thermal shock compared to potash-lime glass, making it less prone to cracking under rapid temperature changes. Potash-lime glass tends to be more fragile due to its lower tensile strength and higher susceptibility to stress-induced fractures. These properties render silica glass ideal for demanding applications requiring durability, while potash-lime glass suits everyday glassware with moderate mechanical requirements.
Cost and Availability Factors
Silica glass, known for its high thermal resistance and chemical durability, is significantly more expensive than potash-lime glass due to its complex manufacturing process and raw material costs. Potash-lime glass dominates the market in terms of availability, benefiting from abundant raw materials and simpler production methods that reduce overall expenses. Cost efficiency and widespread accessibility make potash-lime glass the preferred choice for general glassware, while silica glass is reserved for specialized applications requiring superior durability.
Applications in Laboratory and Household Glassware
Silica glass offers superior thermal resistance and chemical inertness, making it ideal for laboratory glassware such as beakers, flasks, and test tubes that require high durability and precision. Potash-lime glass, being more affordable and easier to manufacture, is commonly used in household glassware like drinking glasses and storage containers where thermal resistance and chemical resistance are less critical. The distinct physical properties of silica glass ensure it withstands high temperatures and corrosive chemicals, whereas potash-lime glass serves well in everyday applications due to its cost-effectiveness and adequate strength.
Choosing the Right Glass: Silica vs Potash-Lime
Silica glass offers superior thermal resistance and chemical durability, making it ideal for high-temperature laboratory and kitchen glassware. Potash-lime glass, while more cost-effective and easier to manufacture, is less resistant to thermal shock and chemical corrosion, suitable for everyday, low-stress applications like drinkware and containers. Selecting silica glass ensures longevity and performance under rigorous conditions, whereas potash-lime glass balances affordability with acceptable durability for general use.
Infographic: Silica glass vs Potash-lime glass for Glassware
 azmater.com
azmater.com