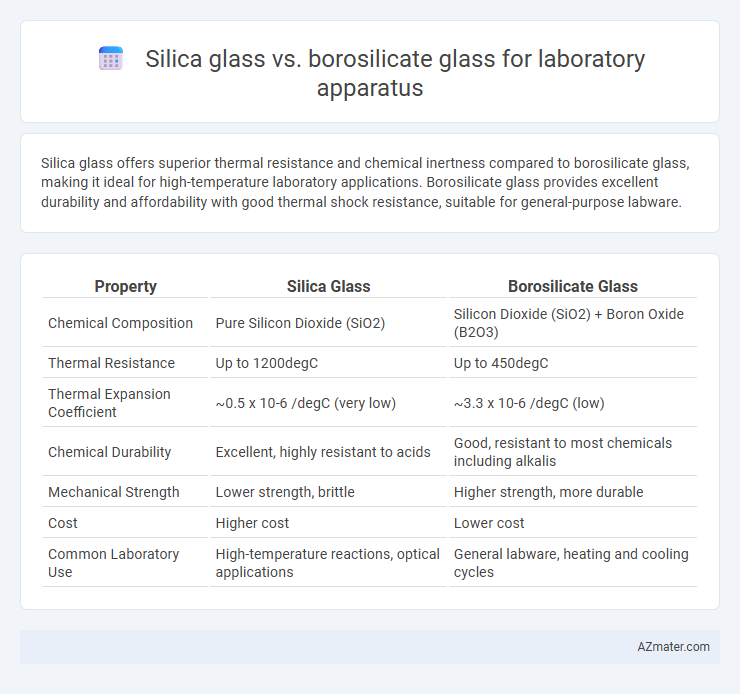
Silica glass offers superior thermal resistance and chemical inertness compared to borosilicate glass, making it ideal for high-temperature laboratory applications. Borosilicate glass provides excellent durability and affordability with good thermal shock resistance, suitable for general-purpose labware.
Table of Comparison
| Property | Silica Glass | Borosilicate Glass |
|---|---|---|
| Chemical Composition | Pure Silicon Dioxide (SiO2) | Silicon Dioxide (SiO2) + Boron Oxide (B2O3) |
| Thermal Resistance | Up to 1200degC | Up to 450degC |
| Thermal Expansion Coefficient | ~0.5 x 10-6 /degC (very low) | ~3.3 x 10-6 /degC (low) |
| Chemical Durability | Excellent, highly resistant to acids | Good, resistant to most chemicals including alkalis |
| Mechanical Strength | Lower strength, brittle | Higher strength, more durable |
| Cost | Higher cost | Lower cost |
| Common Laboratory Use | High-temperature reactions, optical applications | General labware, heating and cooling cycles |
Introduction to Laboratory Glassware Materials
Silica glass, known for its exceptional thermal resistance and chemical inertness, provides high purity and low thermal expansion ideal for precise laboratory applications requiring high-temperature stability. Borosilicate glass, composed of silica and boron trioxide, offers excellent durability, resistance to thermal shock, and chemical corrosion, making it the preferred choice for general laboratory glassware such as beakers and flasks. Both materials serve critical roles in laboratory apparatus, with silica glass favored for high-temperature experiments and borosilicate glass valued for versatility and impact resistance.
Chemical Composition: Silica Glass vs. Borosilicate Glass
Silica glass primarily consists of silicon dioxide (SiO2) with a purity above 99%, offering exceptional thermal stability and chemical resistance. Borosilicate glass is composed of approximately 70-80% silicon dioxide, 7-13% boron oxide (B2O3), along with smaller amounts of sodium oxide and aluminum oxide, enhancing its thermal shock resistance and chemical durability. The addition of boron oxide in borosilicate glass lowers thermal expansion compared to pure silica glass, making it ideal for laboratory apparatus exposed to rapid temperature changes.
Thermal Stability and Resistance Comparison
Silica glass exhibits superior thermal stability with a melting point around 1720degC, making it highly resistant to thermal shock and suitable for high-temperature laboratory applications. Borosilicate glass, with a melting point near 820degC, offers excellent resistance to thermal shock due to its low coefficient of thermal expansion (approximately 3.3 x 10^-6 /degC), which is significantly better than standard soda-lime glass but lower than pure silica glass. For laboratory apparatus requiring extreme thermal resistance and minimal expansion under heat, silica glass outperforms borosilicate glass, although borosilicate remains preferred for general thermal stability and chemical resistance at moderate temperatures.
Mechanical Strength and Durability
Silica glass exhibits superior mechanical strength and higher thermal resistance compared to borosilicate glass, making it ideal for high-stress laboratory applications involving extreme temperatures. Borosilicate glass offers better resistance to chemical corrosion and thermal shock, enhancing its durability in routine experimental use. While silica glass withstands higher mechanical loads, borosilicate glass provides a balanced combination of toughness and resilience, optimizing longevity in standard lab environments.
Chemical Resistance in Laboratory Settings
Silica glass exhibits superior chemical resistance compared to borosilicate glass, making it highly resistant to acids, alkalis, and organic solvents commonly used in laboratory settings. Borosilicate glass offers good chemical resistance but can be more vulnerable to attack by strong alkalis and hydrofluoric acid. For applications involving highly aggressive chemicals, silica glass is preferred due to its enhanced durability and longevity in corrosive environments.
Transparency and Optical Properties
Silica glass exhibits superior transparency across a broad spectrum of wavelengths, including ultraviolet (UV) and infrared (IR) ranges, making it ideal for advanced optical applications in laboratory apparatus. Borosilicate glass offers good transparency but has limited UV transmission compared to silica, restricting its use in experiments requiring UV light exposure. The exceptional optical clarity and low thermal expansion of silica glass enhance precision in measurements and imaging, whereas borosilicate glass provides sufficient clarity with greater chemical and thermal resistance.
Applications in Laboratory Apparatus
Silica glass offers exceptional thermal shock resistance and high chemical purity, making it ideal for high-temperature reactions and UV spectroscopy in laboratory apparatus. Borosilicate glass provides excellent durability against chemical corrosion and moderate thermal resistance, suited for general-purpose laboratory equipment such as beakers, flasks, and condensers. Both materials serve critical roles in laboratory environments, with silica glass preferred for precision applications requiring ultra-pure conditions and borosilicate glass favored for versatile, everyday use.
Cost and Availability Considerations
Silica glass offers superior thermal resistance and chemical durability, but it is significantly more expensive and less widely available compared to borosilicate glass, which is commonly used in laboratory apparatus due to its affordability and easy procurement. Borosilicate glass balances cost-effectiveness with good thermal and chemical stability, making it the preferred choice for most standard lab equipment. Laboratories with budget constraints or high-volume needs often opt for borosilicate glass to maximize both cost efficiency and material availability.
Safety Factors in Laboratory Use
Silica glass exhibits superior thermal shock resistance with a high melting point around 1710degC, reducing the risk of breakage during rapid temperature changes in laboratory use. Borosilicate glass offers excellent chemical durability and low thermal expansion (~3.3 x 10-6 /degC), enhancing safety by minimizing stress fractures under heating and cooling cycles. Both materials ensure safety, but borosilicate's resilience against chemical corrosion makes it more suitable for handling reactive substances, while silica glass withstands extreme temperatures for high-heat applications.
Choosing the Right Glass for Laboratory Needs
Silica glass offers superior thermal shock resistance and high purity, making it ideal for high-temperature applications in laboratory settings, while borosilicate glass provides excellent chemical resistance and durability at a lower cost, suitable for general-purpose labware. When choosing the right glass, consider factors such as thermal tolerance, chemical compatibility, and mechanical strength based on specific experimental requirements. Silica glass is preferred for precise analytical work requiring minimal contamination, whereas borosilicate glass balances performance and affordability for routine lab apparatus.
Infographic: Silica glass vs Borosilicate glass for Laboratory apparatus
 azmater.com
azmater.com