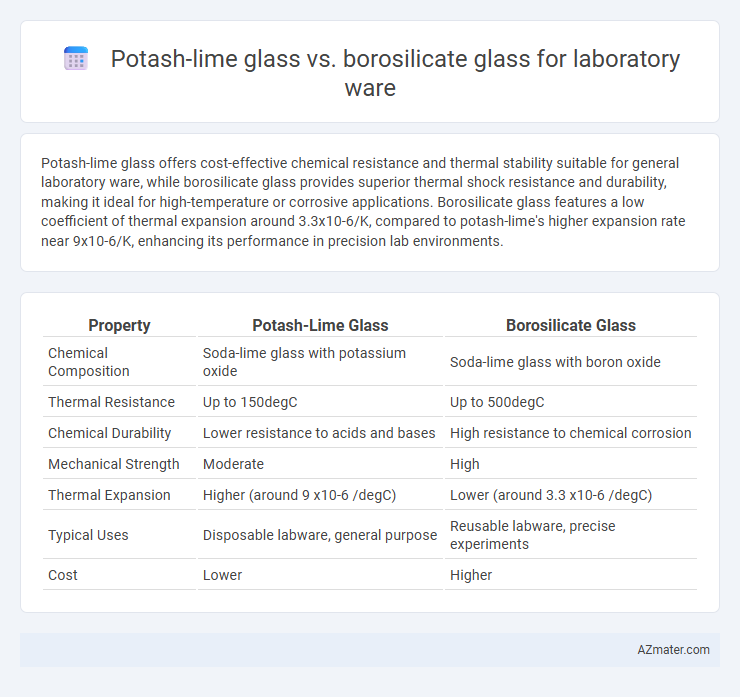
Potash-lime glass offers cost-effective chemical resistance and thermal stability suitable for general laboratory ware, while borosilicate glass provides superior thermal shock resistance and durability, making it ideal for high-temperature or corrosive applications. Borosilicate glass features a low coefficient of thermal expansion around 3.3x10-6/K, compared to potash-lime's higher expansion rate near 9x10-6/K, enhancing its performance in precision lab environments.
Table of Comparison
| Property | Potash-Lime Glass | Borosilicate Glass |
|---|---|---|
| Chemical Composition | Soda-lime glass with potassium oxide | Soda-lime glass with boron oxide |
| Thermal Resistance | Up to 150degC | Up to 500degC |
| Chemical Durability | Lower resistance to acids and bases | High resistance to chemical corrosion |
| Mechanical Strength | Moderate | High |
| Thermal Expansion | Higher (around 9 x10-6 /degC) | Lower (around 3.3 x10-6 /degC) |
| Typical Uses | Disposable labware, general purpose | Reusable labware, precise experiments |
| Cost | Lower | Higher |
Introduction to Laboratory Glassware Materials
Potash-lime glass, composed primarily of silica, potash (K2O), and lime (CaO), offers affordability and ease of manufacturing but has lower thermal and chemical resistance compared to borosilicate glass. Borosilicate glass, enriched with boron oxide (B2O3), provides superior thermal shock resistance and chemical durability, making it ideal for high-precision laboratory applications involving rapid temperature changes or aggressive reagent exposure. Selection between these materials hinges on experimental requirements for thermal stability, chemical inertness, and cost-effectiveness in laboratory ware.
Composition of Potash-Lime Glass
Potash-lime glass used in laboratoryware primarily consists of silica (SiO2), potash (K2O), lime (CaO), and smaller amounts of soda (Na2O) and alumina (Al2O3), providing moderate chemical resistance and thermal stability. The potash content distinguishes it from soda-lime glass by improving resistance to water and mild alkalis, yet it remains less resistant to thermal shock compared to borosilicate glass. Borosilicate glass contains significant amounts of boron oxide (B2O3), granting superior thermal resistance and chemical durability, making it ideal for high-temperature and corrosive laboratory applications.
Composition of Borosilicate Glass
Borosilicate glass, primarily composed of silica (SiO2) and boron trioxide (B2O3), features a unique chemical structure that imparts exceptional thermal resistance and low thermal expansion compared to potash-lime glass. This composition enables it to withstand rapid temperature changes and corrosive chemicals, making it the preferred choice for laboratory ware requiring durability and chemical inertness. Potash-lime glass, with higher alkali and lime content, lacks the enhanced heat resistance that borosilicate glass offers, limiting its use in scientific applications involving extreme conditions.
Thermal Resistance: Potash-Lime vs Borosilicate Glass
Borosilicate glass offers superior thermal resistance compared to potash-lime glass, with a coefficient of thermal expansion around 3.3 x 10^-6 /degC, reducing the risk of cracking under rapid temperature changes. Potash-lime glass, exhibiting a higher thermal expansion coefficient near 9 x 10^-6 /degC, is more prone to thermal shock and less suitable for high-temperature laboratory applications. This makes borosilicate glass the preferred choice for laboratory ware requiring high thermal stability and frequent heating cycles.
Chemical Durability Comparison
Potash-lime glass exhibits lower chemical durability compared to borosilicate glass, making it more susceptible to chemical attack and leaching in acidic or alkaline environments. Borosilicate glass contains boron oxide, which enhances its resistance to thermal shock and chemical corrosion, allowing it to withstand aggressive laboratory reagents. Laboratory ware made from borosilicate glass demonstrates superior longevity and maintains structural integrity under harsh chemical conditions, making it the preferred choice for most chemical applications.
Mechanical Strength and Durability
Potash-lime glass exhibits lower mechanical strength and durability compared to borosilicate glass, making it more prone to breakage under thermal stress or mechanical impact. Borosilicate glass contains silica and boron trioxide, enhancing resistance to thermal shock, chemical corrosion, and mechanical stress, which ensures longer lifespan and reliability in laboratory applications. Therefore, borosilicate glass is preferred for laboratory ware requiring high mechanical strength and durability.
Cost Analysis and Availability
Potash-lime glass is generally more cost-effective and widely available compared to borosilicate glass, making it a preferred choice for routine laboratory ware with low to moderate thermal stress requirements. Borosilicate glass offers superior thermal resistance and chemical durability but comes at a higher price point and may have longer procurement times due to specialized manufacturing processes. Laboratories focusing on budget efficiency and high-volume usage often opt for potash-lime glass, while borosilicate glass is reserved for applications demanding enhanced performance and longevity.
Common Applications in Laboratories
Potash-lime glass is commonly used for inexpensive, disposable laboratory ware such as test tubes, beakers, and flasks in routine chemical experiments due to its moderate thermal resistance and affordability. Borosilicate glass, prized for its superior thermal and chemical resistance, is preferred for precision laboratory applications including high-temperature reactions, reflux apparatus, and storage of aggressive chemicals. Laboratories requiring durable, reusable equipment with high resistance to thermal shock and chemical corrosion predominantly utilize borosilicate glassware.
Safety Considerations for Laboratory Use
Potash-lime glass, commonly used for general laboratory ware, offers good chemical resistance but is more prone to thermal shock and breakage compared to borosilicate glass. Borosilicate glass, with its low coefficient of thermal expansion, provides superior resistance to sudden temperature changes and harsh chemicals, enhancing safety during heating and cooling processes. When prioritizing safety in laboratory environments, borosilicate glass minimizes the risk of accidents caused by glass breakage and chemical corrosion.
Conclusion: Which Glass is Best for Laboratory Ware?
Borosilicate glass outperforms potash-lime glass in laboratory ware due to its superior thermal resistance and chemical durability, making it ideal for experiments involving rapid temperature changes and aggressive chemicals. Potash-lime glass, although less expensive, is more prone to thermal shock and chemical corrosion, limiting its reliability and lifespan in rigorous laboratory settings. Choosing borosilicate glass ensures enhanced safety, longevity, and performance in demanding scientific applications.
Infographic: Potash-lime glass vs Borosilicate glass for Laboratory ware
 azmater.com
azmater.com